|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(CH3)3C-N(H)C(=O)H |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nitrogen |
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
in
N-tert-Butylformamide
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The
low resolution microwave spectrum of each of the following conformers of
N-tert-butylformamide was first observed and assigned by N.S.True
[1], and subsequently revisited at higher resolution by Kannengießer [2].
|
|
|
|
|
|
|
|
|
|
|
|
|
syn OCNC = 0o
|
|
|
anti OCNC = 180o
|
|
|
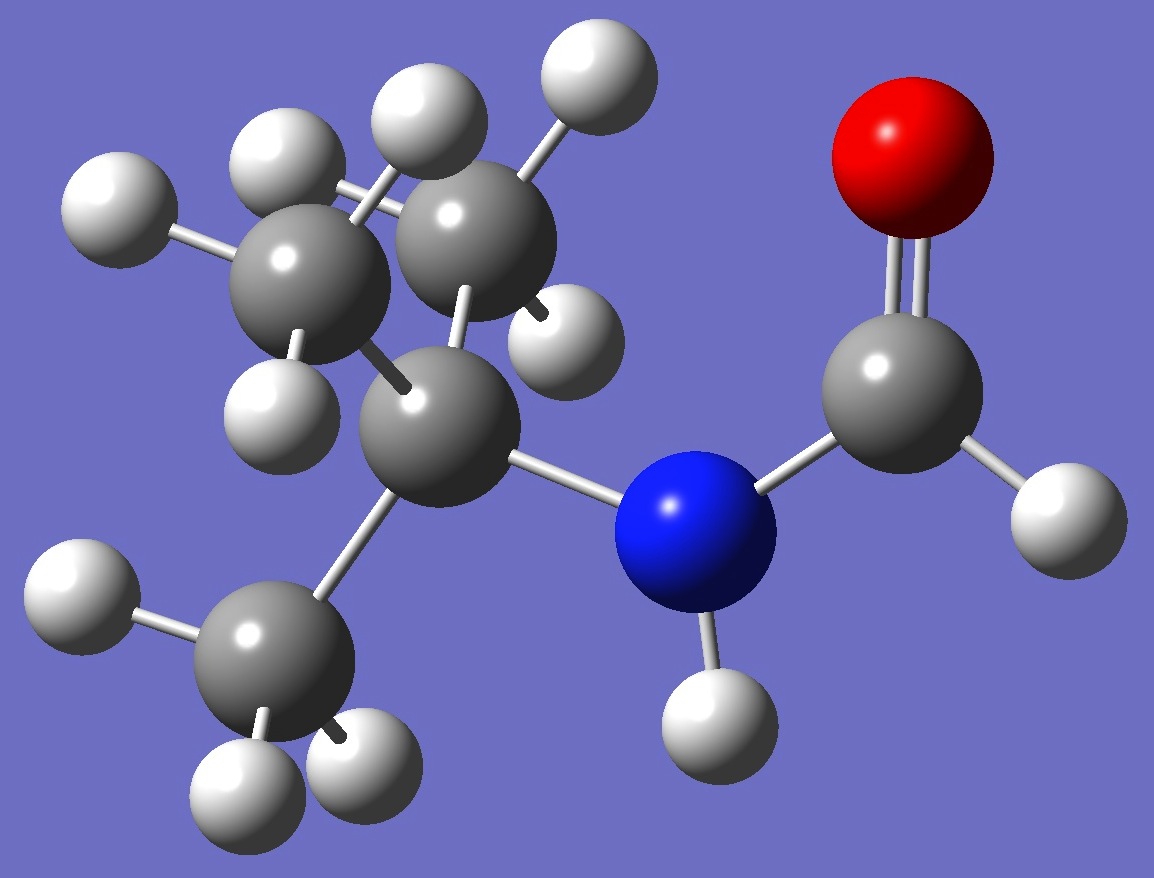
|
|
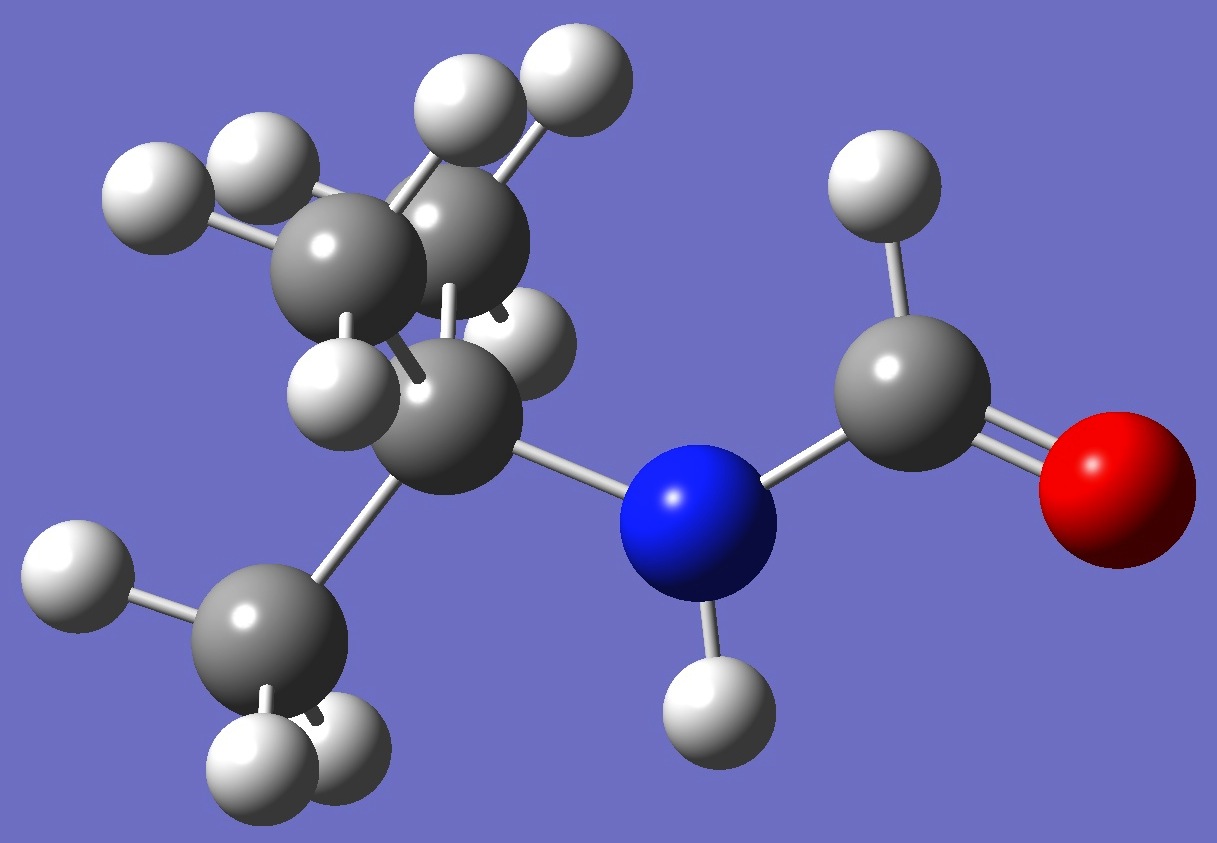
|
|
|
At B3LYP/cc-pVTZ
|
|
|
level of theory,
|
|
|
Esyn < Eanti by |
|
|
7.3 kJ/mole
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Calculation of the
nitrogen nqcc tensor in each conformer was made here on ropt
molecular
structure given by HF/6-311++G(3df,3pd) and MP2/6-311++G(d,p) optimization (assuming Cs
symmetry). These calculated nqcc's are compared with the
experimental values [2] in Tables 1 and 2. Structure parameters
are
given in Table 3, rotational constants and dipole moments in Table 4.
|
|
|
|
|
|
|
|
|
|
|
|
|
In Tables 1 and 2, subscripts a,b,c refer to
the
principal axes of the inertia tensor; x,y,z to the principal axes
of the nqcc tensor. ETA = (Xxx - Xyy)/Xzz.
|
|
|
RSD is the calibration residual standard deviation of
the B3PW91/6-311+G(df,pd) model for calculation of nitrogen efg's/nqcc's. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 1. 14N
nqcc's in syn N-tert-Butylformamide (MHz). Calculation was made on
(1) HF/6-311++G(3df,3pd) and (2) MP2/6-311++G(d,p) optimized
structures. |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc (1)
|
|
Calc (2)
|
|
Expt [2]
|
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
|
1.790
|
|
1.799
|
|
1.80315(71)
|
|
|
Xbb - Xcc |
|
5.862
|
|
5.956
|
|
5.7563(17)
|
|
|
Xbb
|
|
2.036
|
|
2.078
|
|
1.9766(18) *
|
|
|
Xcc |
-
|
3.826
|
-
|
3.878
|
-
|
3.7797(18) *
|
|
|
|Xab| |
|
0.205
|
|
0.093
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RMS
|
|
0.044 (1.8 %)
|
|
0.082 (3.2 %)
|
|
|
|
|
RSD |
|
0.030 (1.3 %) |
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
1.674
|
|
1.771 |
|
|
|
|
Xyy |
|
2.152
|
|
2.106 |
|
|
|
|
Xzz |
-
|
3.826
|
- |
3.878 |
|
|
|
|
ETA |
|
0.125
|
|
0.086 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* Derived here from experimental Xaa and Xbb - Xcc
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 2. 14N
nqcc's in anti N-tert-Butylformamide (MHz). Calculation was made on
(1) HF/6-311++G(3df,3pd) and (2) MP2/6-311++G(d,p) optimized
structures. |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc (1)
|
|
Calc (2)
|
|
Expt [2]
|
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
|
2.125
|
|
2.121
|
|
2.1345(38)
|
|
|
Xbb - Xcc |
|
5.939
|
|
6.064
|
|
5.710(11)
|
|
|
Xbb
|
|
1.907
|
|
1.971
|
|
1.788(12) *
|
|
|
Xcc |
-
|
4.032
|
-
|
4.092
|
-
|
3.922(12) *
|
|
|
|Xab| |
|
0.255
|
|
0.203
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RMS
|
|
0.094 (3.6 %)
|
|
0.144 (5.5 %)
|
|
|
|
|
RSD |
|
0.030 (1.3 %) |
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
1.738
|
|
1.830 |
|
|
|
|
Xyy |
|
2.294
|
|
2.262 |
|
|
|
|
Xzz |
-
|
4.032
|
- |
4.092 |
|
|
|
|
ETA |
|
0.138
|
|
0.106 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* Derived here from experimental Xaa and Xbb - Xcc |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Table 3.
N-tert-Butylformamide. Selected structure parameters, ropt (Å and degrees). Optimized structures (1) HF/6-311++G(3df,3pd) and (2) MP2/6-311++G(d,p). Complete structures are given here is Z-matrix format.
|
|
|
|
|
|
|
|
|
|
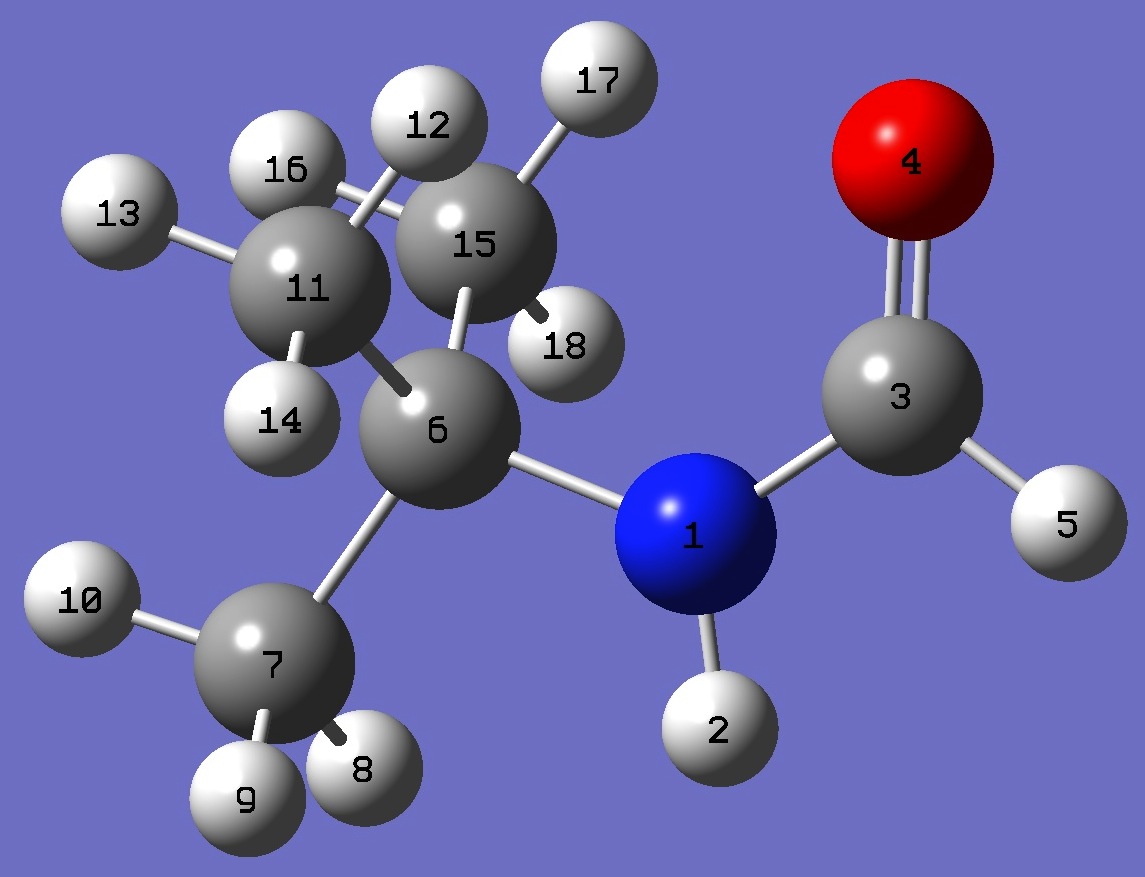
|
syn
|
ropt (1) | ropt (2) |
|
|
|
|
|
C(6)N |
1.4709
|
1.4716
|
|
NC(3) |
1.3419
|
1.3627
|
|
C(3)O |
1.1906
|
1.2224
|
|
C(6)NC(3) |
126.93
|
125.66
|
|
NC(3)O |
126.62
|
125.89
|
|
OC(3)NC(6) |
0.
|
0. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
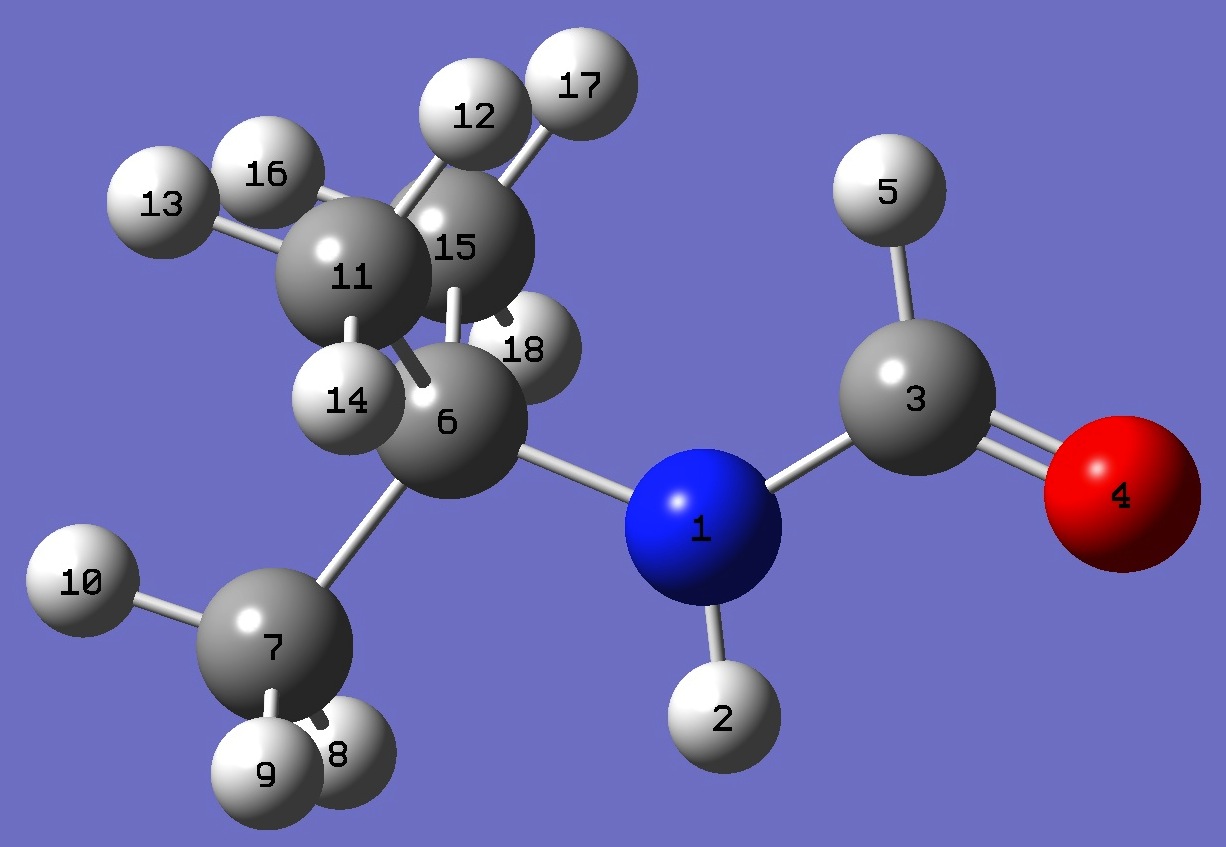
|
anti
|
ropt (1) | ropt (2) |
|
|
|
|
|
C(6)N |
1.4659
|
1.4698
|
|
NC(3) |
1.3421
|
1.3621
|
|
C(3)O |
1.1911
|
1.2228
|
|
C(6)NC(3) |
126.76
|
126.00
|
|
NC(3)O |
124.67
|
124.67
|
|
OC(3)NC(6) |
180.
|
180. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Table 4.
N-tert-Butylformamide (MHz). Rotational constants (MHz). Calculation
was made on
(1) HF/6-311++G(3df,3pd) and (2) MP2/6-311++G(d,p) optimized
structures. |
|
|
|
|
|
|
|
|
Calc (1)
|
Calc (2) |
Expt [2]
|
|
|
|
|
|
|
|
syn
|
A
|
4117
|
4095
|
4079.105661(91)
|
|
|
B
|
1924
|
1924
|
1927.547557(43)
|
|
|
C
|
1842
|
1840
|
1843.423060(41)
|
|
|
|
|
|
|
|
| anti |
A
|
4371
|
4356
|
4331.7(26)
|
|
|
B
|
1598
|
1577
|
1579.85415(40)
|
|
|
C
|
1575
|
1554
|
1558.72921(44)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[1] N.S.True, J.Mol.Struct. 112,333(1984).
|
|
|
[2] Raphaela Kannengießer, "Structures and Dynamics of Formamides,
Acetamides, and Propionamides" Dissertation, RWTH Aachen University,
Institute of Physical Chemistry, 2017.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Formamide |
N-Methylformamide |
N-Ethylformamide
|
|
|
Cyanoformamide
|
N-Vinylformamide
|
N,N-Dimethylformamide
|
|
|
Methyl ethyl formamide
|
cis-Formanilide
|
trans-Formanilide
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Nitrogen |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NtBF.html |
|
|
|
|
|
|
Last
Modified 14 Jan 2018 |
|
|
|
|
|
|
|
|
|
|



