|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
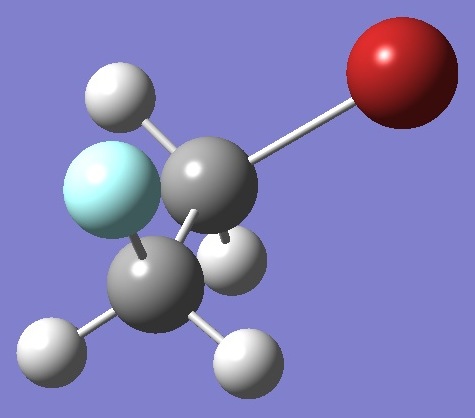
CH2Br-CH2F |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Bromine
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
in gauche 1-Bromo-2-Fluoroethane
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Bromine nqcc's in the gauche conformer of 1-bromo-2-fluoroethane were determined by Niide et al. [1]. |
|
|
|
|
|
|
|
|
|
|
|
|
Calculation of the bromine nqcc tensors was made here on a molecular structure derived ab initio with
bond length correction as discussed below. These are compared
with the experimental nqcc's [1] in Tables 1 and 2. Structure
parameters are given in
Table 3, atomic coordinates in Table 4, and rotational constants in
Table 5. |
|
|
|
|
|
|
|
|
|
|
|
|
In Tables 1 and 2, subscripts a,b,c refer to the principal axes of the inertia
tensor, subscripts x,y,z to the principal axes of the nqcc tensor. Ø (degrees)
is the angle between its subscripted parameters. ETA = (Xxx
- Xyy)/Xzz. |
|
|
RMS is the root mean square
difference between calculated and experimental diagonal nqcc's (percentage of
the average absolute experimental nqcc). RSD is the residual standard deviation
of calibration of the B1LYP/TZV(3df,3p) model for calculation of
the nqcc's. |
|
|
The algebraic
signs of the off-diagonal nqcc's, which depend on the positive/negative
sense of the a,b,c inertia axes, correspond here to the atomic
coordinates given in Table 4. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Table 1. 79Br nqcc's in gauche CH2BrCH2F (MHz). |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc. |
|
Expt. [1] |
|
| |
|
|
|
|
|
|
|
|
79Br |
Xaa |
|
266.57 |
|
266.4(17) |
|
|
|
Xbb |
|
- 12.07 |
|
- 11.8(9) |
|
|
|
Xcc |
- |
254.50 |
- |
254.6(19) |
|
|
|
Xab |
+ |
394.38 |
|
392.7(43) * |
|
|
|
Xac |
- |
117.84 |
|
|
|
|
|
Xbc |
|
- 91.51 |
|
|
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
0.2 (0.11 %) |
|
|
|
|
|
RSD |
|
1.58 (0.39 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
- |
278.05 |
|
|
|
|
|
Xyy |
- |
294.34 |
|
|
|
|
|
Xzz |
|
572.39 |
|
|
|
|
|
ETA |
|
0.0284 |
|
|
|
|
|
Øz,CBr |
|
0.48 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* Absolute value. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Table 2. 81Br nqcc's in gauche CH2BrCH2F (MHz). |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc. |
|
Expt. [1] |
|
| |
|
|
|
|
|
|
|
|
81Br |
Xaa |
|
223.45 |
|
225.5(12) |
|
|
|
Xbb |
|
- 10.76 |
|
- 11.5(7) |
|
|
|
Xcc |
- |
212.69 |
- |
214.0(14) |
|
|
|
Xab |
+ |
329.28 |
|
336.5(31) * |
|
|
|
Xac |
|
- 98.39 |
|
|
|
|
|
Xbc |
|
- 76.25 |
|
|
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
1.5 (0.97 %) |
|
|
|
|
|
RSD |
|
1.38 (0.40 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
- |
232.30 |
|
|
|
|
|
Xyy |
- |
245.91 |
|
|
|
|
|
Xzz |
|
478.21 |
|
|
|
|
|
ETA |
|
0.0284 |
|
|
|
|
|
Øz,CBr |
|
0.48 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* Absolute value. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Molecular Structure |
|
|
|
|
|
|
|
|
|
|
|
|
The molecular structure was
optimized
at the MP2/6-311+G(d,p) level of theory. The optimized C-C and
C-F bond lengths respectively were corrected using equations obtained
from linear regression of the data given in references [2] and
[3]. The CH bond
lengths were corrected using r = 1.001 ropt, where ropt
is obtained by MP2/6-31G(d,p) optimization [4]. Interatomic
angles are those given by MP2/6-311+G(d,p)
optimization. |
|
|
For the C-Br bond length,
optimization was made at the MP2/6-311+G(2d,p) level of theory of the
C-Br equilibrium bond lengths in CH3Br, CH2Br2,
HCCBr, and BrCN. Linear regression of the calculated versus
equilibrium bond lengths yields the following relationship, by which
the C-Br was corrected: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
r = 0.9946 × ropt + 0.0001, RSD = 0.0015 Å. |
|
|
|
|
|
|
|
|
|
|
|
|
The optimized C-Br bond length is 1.9446 Å which, after correction is 1.934 Å. |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
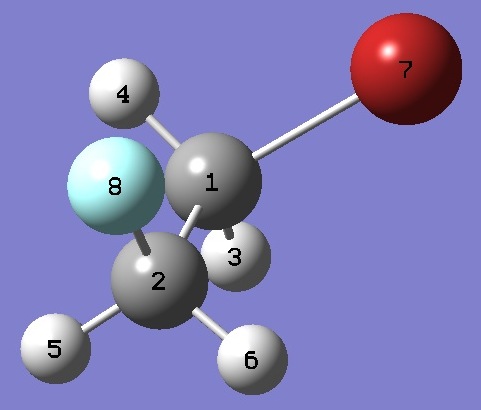
| Table 3.
Gauche 1-Bromo-2-Fluoroethane. Heavy atom structure parameters
(Å and degrees). The complete structure is given here in Z-Matrix format. |
| |
|
|
|
|
|
|
BrC(1) |
1.934 |
| C(1)C(2) |
1.503 |
| C(2)F |
1.3785 |
| C(2)C(1)Br |
112.03 |
| C(1)C(2)F |
110.40 |
| BrC(1)C(2)F |
66.51 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Table 4.
Gauche 1-Bromo-2-Fluoroethane. Atomic coordinates. Normal species. |
| |
|
|
|
|
|
|
|
|
|
|
a (Å) |
|
b (Å) |
|
c (Å) |
|
|
|
|
|
|
|
|
|
C(1) |
|
0.5895 |
|
1.0015 |
- |
0.3065 |
|
C(2) |
|
1.8149 |
|
0.4743 |
|
0.3858 |
|
H(3) |
|
0.3599 |
|
1.9985 |
|
0.0638 |
|
H(4) |
|
0.7432 |
|
1.0338 |
- |
1.3821 |
|
H(5) |
|
2.6407 |
|
1.1767 |
|
0.2386 |
|
H(6) |
|
1.6401 |
|
0.3250 |
|
1.4523 |
|
Br |
- |
0.9620 |
- |
0.1055 |
|
0.0215 |
|
F |
|
1.1918 |
- |
0.7346 |
- |
0.1591 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
| Table 5. Gauche 1-Bromo-2-Fluoroethane. Rotational constants (MHz). Normal species. |
| |
|
|
|
|
|
Calc. ropt |
Expt. [1] |
|
|
|
|
|
A |
12 630.3 |
12 547.013(44) |
|
B |
2 236.4 |
2 218.112(7) |
|
C |
2 014.2 |
1 998.444(7) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[1] Y.Niide, I.Ohkoshi, M.Takano, and M.Hayashi, J.Mol.Spectrosc. 115,305(1986). |
|
|
[2] J.Demaison, J.Cosléou, R.Bocquet, and A.G.Lesarri, J.Mol.Spectrosc. 167,400(1994). |
|
|
[3] R.M.Villamañan, W.D.Chen,
G.Wlodarczak, J.Demaison, A.G.Lesarri, J.C.López, and
J.L.Alonso, J.Mol.Spectrosc. 171,223(1995). |
|
|
[4] J.Demaison and G.Wlodarczak, Structural Chem. 5,57(1994). |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ethyl Bromide |
1-Bromo-1-Fluoroethane |
|
|
|
Methyl Bromide |
2-Bromoethanol |
|
|
|
|
2-Bromopropane |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Bromine |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
gCH2BrCH2F.html |
|
|
|
|
|
|
Last
Modified 7 Aug 2006 |
|
|
|
|
|
|
|
|
|
|

