|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
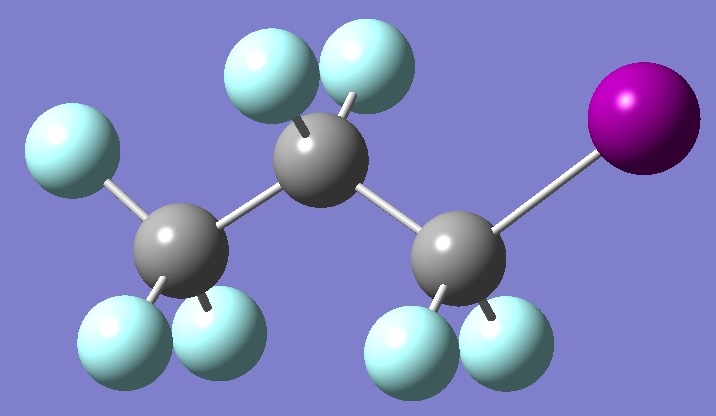
CF3-CF2-CF2I
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Iodine |
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
in
trans-1-Iodoperfluoropropane
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The 127I nqcc tensor in
t-1-iodoperfluoropropane has been determined by Dewberry et
al. [1].
|
|
|
|
|
|
|
|
|
|
|
|
|
Calculation of the 127I
nqcc tensor
in 1-Iodoperfluoropropane was made here on molecular
structures
given by MP2/6-311+G(d,p),
MP2/6-311+G(2d,p), MP2/6-311+G(3d,3p), and MP2/6-311G(3df,3pd)
optimizations; and on these
same structures but with empirically
corrected
C-C and CF bond lengths. These calculated nqcc's are given
in Tables 1 - 4. Structure
parameters are given in Table 5, rotational constants in Table 6. |
|
|
|
|
|
|
|
|
|
|
|
|
In Tables 1 - 4, subscripts a,b,c
refer to the principal axes of the inertia tensor; x,y,z to the
principal axes of the nqcc tensor. Ø (degrees) is the
angle between its subscripted parameters. ETA = (Xxx -
Xyy)/Xzz. |
|
|
RMS is the root mean square
difference between calculated and experimental diagonal nqcc's (percent
of average of absolute experimental nqcc's). RSD is the
calibration residual
standard deviation of
the B1LYP/6-311G(df,p) model for calculation of the nqcc's, which may
be taken as an estimate of the uncertainty in the calculated nqcc's. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 1. 127I
nqcc's in t-1-Iodoperfluoropropane (MHz). |
|
|
|
|
|
|
|
|
|
|
|
Calc (1) was made on the
MP2/6-311+G(d,p) optimized molecular structure. |
|
|
Calc (2) was made on this same
structure but with empirically corrected C-C and CF bond lengths. |
|
|
|
|
|
|
|
|
|
|
|
|
|
Calc (1) |
|
Calc (2) |
|
Expt [1] |
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
- |
1800.6 |
- |
1793.1 |
- |
1798.4013(58) |
|
|
Xbb |
|
719.7 |
|
719.6 |
|
716.0883(62) |
|
|
Xcc |
|
1080.9 |
|
1073.5 |
|
1082.313(11) |
|
|
|Xab| |
|
990.1 |
|
976.3 |
|
991.7058(35) |
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
2.6 (0.21 %) |
|
6.3 (0.52 %) |
|
|
|
|
RSD |
|
15.2 (1.23 %) |
|
15.2 (1.23 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
1062.1 |
|
1054.4 |
|
1060.1382(60) |
|
|
Xyy |
|
1080.9 |
|
1073.5 |
|
1082.3130(11) |
|
|
Xzz |
- |
2143.0 |
- |
2127.9 |
- |
2142.4512(49) |
|
|
ETA |
|
0.0088 |
|
0.0090 |
|
0.01035(7) |
|
|
Øz,a |
|
19.08 |
|
18.92 |
|
19.133 |
|
|
Øa,CI |
|
21.26 |
|
20.18 |
|
|
|
|
Øz,CI |
|
2.18 |
|
2.26 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 2. 127I
nqcc's in t-1-Iodoperfluoropropane (MHz). |
|
|
|
|
|
|
|
|
|
|
|
Calc (1) was made on the
MP2/6-311+G(2d,p) optimized molecular structure. |
|
|
Calc (2) was made on this same
structure but with empirically corrected C-C and CF bond lengths. |
|
|
|
|
|
|
|
|
|
|
|
|
|
Calc (1) |
|
Calc (2) |
|
Expt. |
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
- |
1782.8 |
- |
1771.0 |
- |
1798.4013(58) |
|
|
Xbb |
|
711.2 |
|
708.1 |
|
716.0883(62) |
|
|
Xcc |
|
1071.6 |
|
1063.0 |
|
1082.313(11) |
|
|
|Xab| |
|
981.9 |
|
968.6 |
|
991.7058(43) |
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
11.3 (0.94 %) |
|
19.9 (1.66 %) |
|
|
|
|
RSD |
|
15.2 (1.23 %) |
|
15.2 (1.23 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
1051.3 |
|
1041.6 |
|
1060.1382(60) |
|
|
Xyy |
|
1071.6 |
|
1063.0 |
|
1082.3130(11) |
|
|
Xzz |
- |
2122.9 |
- |
2104.6 |
- |
2142.4512(49) |
|
|
ETA |
|
0.0095 |
|
0.0102 |
|
0.01035(7) |
|
|
Øz,a |
|
19.11 |
|
19.00 |
|
19.133 |
|
|
Øa,CI |
|
21.28 |
|
21.28 |
|
|
|
|
Øz,CI |
|
2.18 |
|
2.28 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 3. 127I
nqcc's in t-1-Iodoperfluoropropane (MHz). |
|
|
|
|
|
|
|
|
|
|
|
Calc (1) was made on the
MP2/6-311+G(3d,3p) optimized molecular structure. |
|
|
Calc (2) was made on this same
structure but with empirically corrected C-C and CF bond lengths. |
|
|
|
|
|
|
|
|
|
|
|
|
|
Calc (1) |
|
Calc (2) |
|
Expt [1] |
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
- |
1787.3 |
- |
1777.1 |
- |
1798.4013(58) |
|
|
Xbb |
|
711.8 |
|
710.2 |
|
716.0883(62) |
|
|
Xcc |
|
1075.4 |
|
1066.9 |
|
1082.313(11) |
|
|
|Xab| |
|
991.0 |
|
976.6 |
|
991.7058(43) |
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
7.9 (0.66 %) |
|
15.5 (1.30 %) |
|
|
|
|
RSD |
|
15.2 (1.23 %) |
|
15.2 (1.23 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
1057.1 |
|
1047.8 |
|
1060.1382(60) |
|
|
Xyy |
|
1075.4 |
|
1066.9 |
|
1082.3130(11) |
|
|
Xzz |
- |
2132.5 |
- |
2114.8 |
- |
2142.4512(49) |
|
|
ETA |
|
0.0086 |
|
0.0090 |
|
0.01035(7) |
|
|
Øz,a |
|
19.21 |
|
19.07 |
|
19.133 |
|
|
Øa,CI |
|
21.35 |
|
21.30 |
|
|
|
|
Øz,CI |
|
2.14 |
|
2.23 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 4. 127I
nqcc's in t-1-Iodoperfluoropropane (MHz). |
|
|
|
|
|
|
|
|
|
|
|
Calc (1) was made on the
MP2/6-311G(3df,3pd) optimized molecular structure. |
|
|
Calc (2) was made on this same
structure but with empirically corrected C-C and CF bond lengths. |
|
|
|
|
|
|
|
|
|
|
|
|
|
Calc (1) |
|
Calc (2) |
|
Expt. |
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
- |
1752.2 |
- |
1754.2 |
- |
1798.4013(58) |
|
|
Xbb |
|
693.7 |
|
695.4 |
|
716.0883(62) |
|
|
Xcc |
|
1058.4 |
|
1058.8 |
|
1082.313(11) |
|
|
|Xab| |
|
984.3 |
|
983.4 |
|
991.7058(43) |
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
32.7 (2.73 %) |
|
31.2 (2.61 %) |
|
|
|
|
RSD |
|
15.2 (1.23 %) |
|
15.2 (1.23 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
1040.6 |
|
1041.4 |
|
1060.1382(60) |
|
|
Xyy |
|
1058.4 |
|
1058.8 |
|
1082.3130(11) |
|
|
Xzz |
- |
2099.0 |
- |
2100.2 |
- |
2142.4512(49) |
|
|
ETA |
|
0.0085 |
|
0.0083 |
|
0.01035(7) |
|
|
Øz,a |
|
19.41 |
|
19.38 |
|
19.133 |
|
|
Øa,CI |
|
21.52 |
|
21.48 |
|
|
|
|
Øz,CI |
|
2.11 |
|
2.10 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
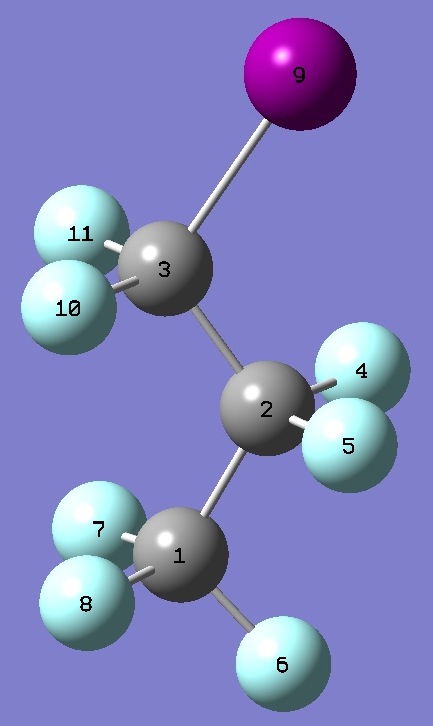
| Table 5.
t-1-Iodoperfluoropropane. Selected structure
parameters (Å and
degrees). Complete structures are given here in Z-matrix representation. |
| |
|
|
|
|
r (1) = MP2/6-311+G(d,p) opt |
|
r (2) = r (1) with
corrected C-C and CF bond lengths. |
|
|
|
|
| Point Group CS |
|
r (1) |
r (2) |
|
|
|
|
|
IC(3) |
2.1647 |
2.1647 |
| C(3)C(2) |
1.5473 |
1.5382 |
| C(2)C(1) |
1.5498 |
1.5406 |
| C(1)F(6) |
1.3323 |
1.3254 |
| IC(3)C(2) |
111.27 |
111.27 |
| C(3)C(2)C(1) |
115.33 |
115.33 |
| C(2)C(1)F(6) |
108.46 |
108.46 |
| |
|
|
| r (1) = MP2/6-311+G(2d,p) opt |
| r (2) = r (1) with
corrected C-C and CF bond lengths. |
|
|
|
|
r (1) |
r (2) |
|
|
|
| IC(3) |
2.1374 |
2.1374 |
| C(3)C(2) |
1.5395 |
1.5382 |
| C(2)C(1) |
1.5437 |
1.5406 |
| C(1)F(6) |
1.3327 |
1.3254 |
| IC(3)C(2) |
111.48 |
111.48 |
| C(3)C(2)C(1) |
115.43 |
115.43 |
| C(2)C(1)F(6) |
108.36 |
108.36 |
| |
|
|
|
r (1) = MP2/6-311+G(3d,3p) opt |
|
r (2) = r (1) with
corrected C-C and CF bond lengths. |
|
|
|
|
|
|
r (1) |
r (2) |
|
|
|
|
|
IC(3) |
2.1539 |
2.1539 |
|
C(3)C(2) |
1.5437 |
1.5382 |
|
C(2)C(1) |
1.5470 |
1.5406 |
|
C(1)F(6) |
1.3332 |
1.3254 |
|
IC(3)C(2) |
111.03 |
111.03 |
|
C(3)C(2)C(1) |
115.09 |
115.09 |
|
C(2)C(1)F(6) |
108.38 |
108.38 |
|
|
|
|
|
r (1) = MP2/6-311G(3df,3pd) opt |
|
r (2) = r (1) with
corrected C-C and CF bond lengths. |
|
|
|
|
|
|
r (1) |
r (2) |
|
|
|
|
|
IC(3) |
2.1414 |
2.1414 |
|
C(3)C(2) |
1.5431 |
1.5382 |
|
C(2)C(1) |
1.5462 |
1.5406 |
|
C(1)F(6) |
1.3254 |
1.3254 |
|
IC(3)C(2) |
110.58 |
110.58 |
|
C(3)C(2)C(1) |
114.72 |
114.72 |
|
C(2)C(1)F(6) |
108.33 |
108.33 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Table 6.
t-1-Iodoperfluoropropane. Rotational constants
(MHz). |
| |
|
|
|
|
|
r (1) = MP2/6-311+G(d,p) opt |
|
r (2) = r (1) with
corrected C-C and CF bond lengths. |
|
|
|
|
|
|
|
r (1) |
r (2) |
Expt [1] |
|
|
|
|
|
|
A |
1567.7 |
1584.2 |
1572.127966(99) |
|
B |
396.0 |
398.9 |
398.458568(34) |
|
C |
380.6 |
383.3 |
382.831125(34) |
| |
|
|
|
|
|
r (1) = MP2/6-311+G(2d,p) opt |
|
r (2) = r (1) with
corrected C-C and CF bond lengths. |
|
|
|
|
|
|
|
r (1) |
r (2) |
Expt [1] |
|
|
|
|
|
|
A |
1572.7 |
1587.7 |
1572.127966(99) |
|
B |
401.0 |
402.3 |
398.458568(34) |
|
C |
385.8 |
387.0 |
382.831125(34) |
| |
|
|
|
|
|
r (1) = MP2/6-311+G(3d,3p) opt |
|
r (2) = r (1) with
corrected C-C and CF bond lengths. |
|
|
|
|
|
|
|
r (1) |
r (2) |
Expt [1] |
|
|
|
|
|
|
A |
1566.6 |
1583.3 |
1572.127966(99) |
|
B |
399.1 |
401.2 |
398.458568(34) |
|
C |
383.7 |
385.7 |
382.831125(34) |
|
|
|
|
|
|
r (1) = MP2/6-311G(3df,3pd) opt |
|
r (2) = r (1) with
corrected C-C and CF bond lengths. |
|
|
|
|
|
|
|
r (1) |
r (2) |
Expt [1] |
|
|
|
|
|
|
A |
1579.5 |
1581.3 |
1572.127966(99) |
|
B |
403.4 |
404.6 |
398.458568(34) |
|
C |
387.6 |
388.8 |
382.831125(34) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[1] C.T.Dewberry, G.S.Grubbs II, and
S.A.Cooke, Abstract, 64th Ohio State University Symposium on Molecular
Spectroscopy, June 22-26, 2009; J.Mol.Spectrosc. 257,66(2009). |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
t-CH3CH2CH2I |
g-CH3CH2CH2I |
CH3I |
CH3CH2I |
|
|
g-CH2ICH2F |
CH3-O-CH2I |
CH3CHICH3 |
(CH3)2CHCH2I |
|
|
t-CF3CH2CH2I |
t-CF3CF2CH2I |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Iodine |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
tCF3CF2CF2I.html |
|
|
|
|
|
|
Last
Modified 25 June 2009 |
|
|
|
|
|
|
|
|
|
|

