|
| |
|
|
|
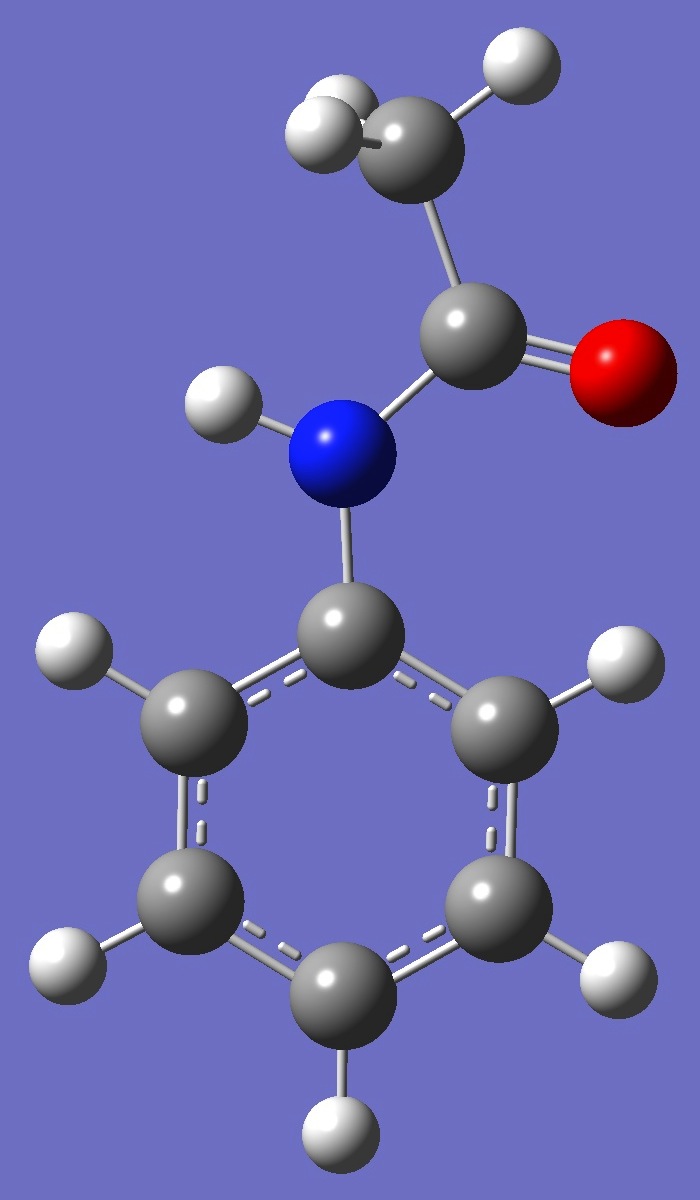
Table 2. Acetanilide.
Structure parameters (Ĺ and
degrees).
|
| |
|
|
|
|
ropt (1) =
HF/6-311+G(d,p) optimization. |
|
ropt (2) =
HF/6-311++G(3df,3pd) optimization. |
|
|
|
|
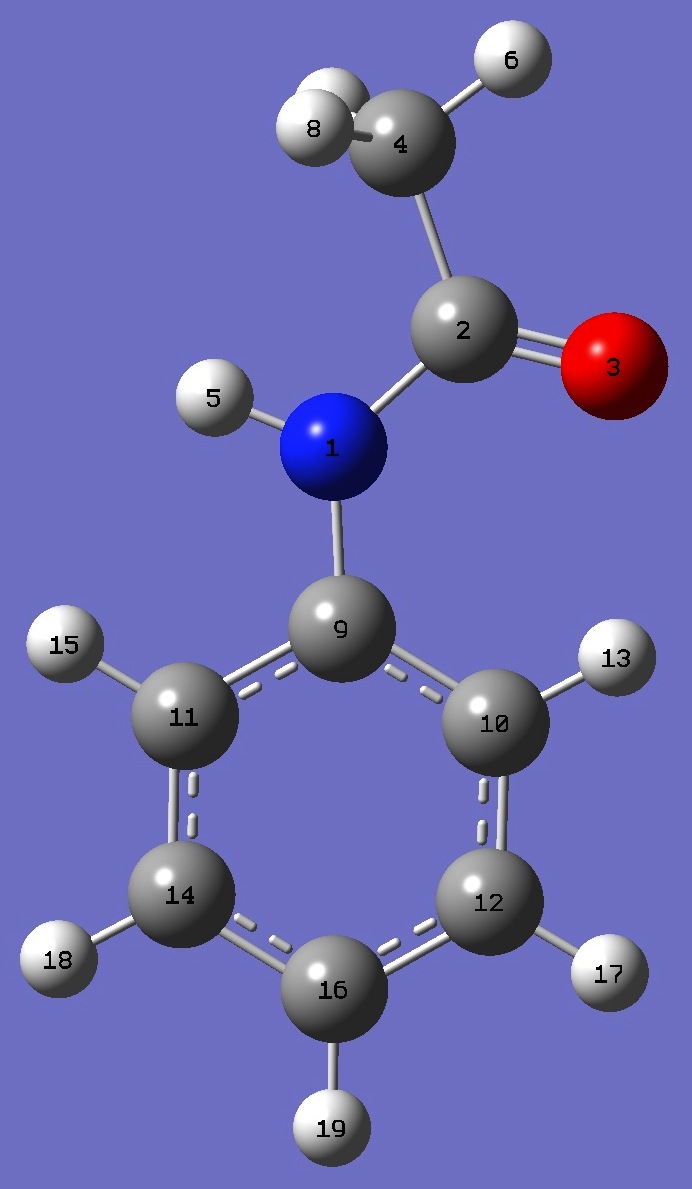
|
N
C,1,B1
O,2,B2,1,A1
C,2,B3,1,A2,3,D1,0
H,1,B4,2,A3,3,D2,0
H,4,B5,2,A4,1,D3,0
H,4,B6,2,A5,1,D4,0
H,4,B7,2,A6,1,D5,0
C,1,B8,2,A7,3,D6,0
C,9,B9,1,A8,2,D7,0
C,9,B10,1,A9,2,D8,0
C,10,B11,9,A10,1,D9,0
H,10,B12,9,A11,1,D10,0
C,11,B13,9,A12,1,D11,0
H,11,B14,9,A13,1,D12,0
C,14,B15,11,A14,9,D13,0
H,12,B16,10,A15,9,D14,0
H,14,B17,11,A16,9,D15,0
H,16,B18,14,A17,11,D16,0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ropt (1) |
ropt (2) |
|
|
|
|
|
B1=1.36525997
B2=1.19264516
B3=1.51441203
B4=0.99277855
B5=1.07970096
B6=1.08582905
B7=1.08582905
B8=1.40912719
B9=1.38968931
B10=1.39299231
B11=1.3870695
B12=1.0686616
B13=1.38101153
B14=1.07708392
B15=1.38574257
B16=1.07565629
B17=1.0755183
B18=1.07477692
A1=124.22677931
A2=113.60176467
A3=115.84616791
A4=108.67743428
A5=110.53750606
A6=110.53750606
A7=129.39635062
A8=123.8154982
A9=116.86553858
A10=119.32153931
A11=120.22237736
A12=120.6417535
A13=119.79358525
A14=120.32388988
A15=118.6339602
A16=119.37942342
A17=120.52177375
D1=180.
D2=180.
D3=180.
D4=-59.94428519
D5=59.94428519
D6=0.
D7=0.
D8=180.
D9=180.
D10=0.
D11=180.
D12=0.
D13=0.
D14=180.
D15=180.
D16=180.
|
B1=1.36151038
B2=1.19025929
B3=1.5129455
B4=0.9904733
B5=1.07710392
B6=1.08347705
B7=1.08347705
B8=1.40511397
B9=1.38661287
B10=1.38981353
B11=1.38354786
B12=1.06606494
B13=1.37745006
B14=1.07471283
B15=1.38226635
B16=1.07327003
B17=1.0731319
B18=1.0722807
A1=124.28886368
A2=113.60653196
A3=115.68547705
A4=108.73332872
A5=110.49802357
A6=110.49802357
A7=129.56135608
A8=123.85240915
A9=116.87531082
A10=119.31739792
A11=120.19669192
A12=120.66770516
A13=119.74302142
A14=120.35026675
A15=118.61137419
A16=119.37256531
A17=120.54845804
D1=180.
D2=180.
D3=180.
D4=-59.86329779
D5=59.86329779
D6=0.
D7=0.
D8=180.
D9=180.
D10=0.
D11=180.
D12=0.
D13=0.
D14=180.
D15=180.
D16=180.
|
|
|
|
|
|
|
|