|
| |
|
|
Table 2. 1-Iodohexane GAAA. ropt (1) = MP2/6-311G(2d,2p) optimization, and ropt (2) = MP2/6-311G(df,pd) structure parameters
(Å and degrees).
|
| |
|
|
| |
|
|
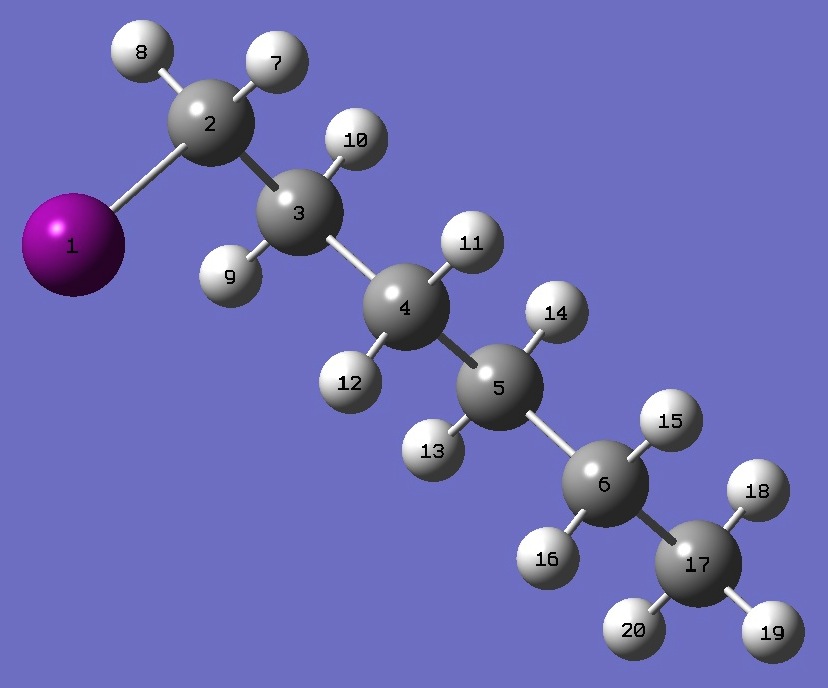 |
I
C,1,B1
C,2,B2,1,A1
C,3,B3,2,A2,1,D1,0
C,4,B4,3,A3,2,D2,0
C,5,B5,4,A4,3,D3,0
H,2,B6,1,A5,3,D4,0
H,2,B7,1,A6,3,D5,0
H,3,B8,2,A7,1,D6,0
H,3,B9,2,A8,1,D7,0
H,4,B10,3,A9,2,D8,0
H,4,B11,3,A10,2,D9,0
H,5,B12,4,A11,3,D10,0
H,5,B13,4,A12,3,D11,0
H,6,B14,5,A13,4,D12,0
H,6,B15,5,A14,4,D13,0
C,6,B16,5,A15,4,D14,0
H,17,B17,6,A16,5,D15,0
H,17,B18,6,A17,5,D16,0
H,17,B19,6,A18,5,D17,0
|
|
|
|
|
ropt (1) |
ropt (2) |
|
|
|
|
B1=2.14734772
B2=1.51642712
B3=1.5228648
B4=1.52471271
B5=1.52521212
B6=1.08475491
B7=1.08329881
B8=1.08976553
B9=1.09333789
B10=1.0926008
B11=1.09016482
B12=1.09232955
B13=1.09248708
B14=1.09077589
B15=1.09057336
B16=1.52555348
B17=1.08839651
B18=1.08717493
B19=1.08833167
A1=113.12967476
A2=114.28039946
A3=112.61672936
A4=112.96301403
A5=105.47212229
A6=104.90120166
A7=109.75060826
A8=106.95180276
A9=109.18097941
A10=109.51627908
A11=109.23308805
A12=109.56610454
A13=109.11188967
A14=109.05174577
A15=112.53161935
A16=110.7840785
A17=111.67871205
A18=110.755929
D1=-66.05552202
D2=-176.53011644
D3=-179.58614405
D4=-122.37772209
D5=122.47422454
D6=57.97827835
D7=173.46097802
D8=-54.76538304
D9=61.59065328
D10=-57.82585501
D11=58.37406313
D12=-58.09961381
D13=57.75536802
D14=179.83076883
D15=59.70187364
D16=179.99643495
D17=-59.72882593
|
B1=2.14058788
B2=1.51886716
B3=1.52202133
B4=1.52366416
B5=1.52389305
B6=1.09038308
B7=1.08902278
B8=1.0943919
B9=1.09772897
B10=1.09686918
B11=1.09427001
B12=1.09657887
B13=1.09672674
B14=1.09520338
B15=1.09501071
B16=1.52363376
B17=1.09245026
B18=1.09144124
B19=1.0923931
A1=113.11185879
A2=114.41350331
A3=112.6980773
A4=113.05039204
A5=105.87510958
A6=105.28473638
A7=109.65071937
A8=106.76183346
A9=109.09972934
A10=109.44094656
A11=109.1285399
A12=109.43954259
A13=108.93501086
A14=108.88712453
A15=112.66777894
A16=110.65780766
A17=111.4935578
A18=110.63067513
D1=-65.85523293
D2=-176.01845009
D3=-179.20153935
D4=-122.1437649
D5=122.21901171
D6=58.00446702
D7=173.72874433
D8=-54.38519587
D9=62.21774504
D10=-57.50167906
D11=58.88794173
D12=-58.0666617
D13=57.89112276
D14=179.92160116
D15=59.77216519
D16=-179.97744481
D17=-59.743583
|
|
|
|
|
|