|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CF3-CH2-CHI-CH3
|
|
|
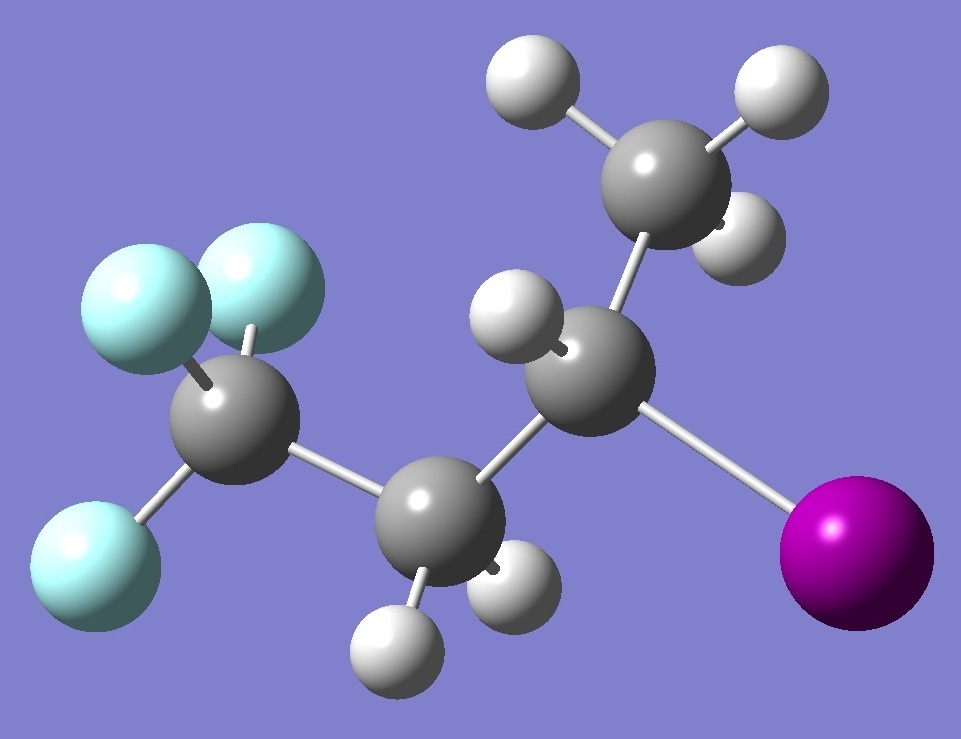
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Iodine |
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
in
tg-3-Iodo-1,1,1-trifluorobutane
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Calculation of the 127I
nqcc tensor
in tg-1-Iodo-3,3,3-trifluoropropane
was made here on molecular structures
given by MP2/6-311+G(d,p),
MP2/6-311+G(2d,2p), MP2/6-311+G(3d,3p), and MP2/6-311+G(df,pd)
optimizations; and on these
same structures but with empirically
corrected
C-C, CF, and CH bond lengths. These calculated nqcc's are
compared with experimental values
in Tables 1 - 4. Structure
parameters are given in Table 5, rotational constants in Table 6. |
|
|
|
|
|
|
|
|
|
|
|
|
In Tables 1 - 4, subscripts a,b,c
refer to the principal axes of the inertia tensor; x,y,z to the
principal axes of the nqcc tensor. Øz,CI
(degrees) is the
angle between the z- and CI bond axes. ETA = (Xxx -
Xyy)/Xzz. |
|
|
RMS is the root mean square
difference between calculated and experimental diagonal nqcc's (percent
of the average of the absolute experimental nqcc's). RSD is the
calibration residual
standard deviation of
the B1LYP/6-311G(df,p) model for calculation of the nqcc's, which may
be taken as an estimate of the uncertainty in the calculated nqcc's. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 1. 127I
nqcc's in tg-3-Iodo-1,1,1-trifluorobutane
(MHz). |
|
|
|
|
|
|
|
|
|
|
|
Calc (1) was made on the
MP2/6-311+G(d,p) optimized molecular structure. |
|
|
Calc (2) was made on this same
structure but with empirically corrected C-C, CF, and CH bond lengths. |
|
|
|
|
|
|
|
|
|
|
|
|
|
Calc (1) |
|
Calc (2) |
|
Expt [1] |
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
- |
1495.8 |
- |
1491.8 |
- |
1473.890(40) |
|
|
Xbb |
|
672.7 |
|
672.3 |
|
656.689(46) * |
|
|
Xcc |
|
823.1 |
|
819.4 |
|
817.2013(236) |
|
|
Xab |
|
788.8 |
|
785.1 |
|
801.934(44) ** |
|
|
Xac |
|
450.8 |
|
450.6 |
|
445.207(67) ** |
|
|
Xbc |
|
- 146.8 |
|
- 145.8 |
|
- 149.799(35) ** |
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
16.0 (1.63 %)
|
|
13.8 (1.40 %)
|
|
|
|
|
RSD |
|
15.2 (1.23 %) |
|
15.2 (1.23 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
907.6 |
|
904.0 |
|
900.662(33) |
|
|
Xyy |
|
929.3 |
|
927.1 |
|
924.826(50) |
|
|
Xzz |
- |
1836.9 |
- |
1831.2 |
- |
1825.488(49) |
|
|
ETA |
|
0.01180 |
|
0.01262 |
|
0.01324(3) |
|
|
Øz,CI |
|
0.53 |
|
0.57 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* Calculated here from zero trace
condition of the nqcc tensor. This note applies also to Tables 2
- 4. |
|
|
** Algebraic signs taken from
calculated values. This note applies also to Tables 2
- 4. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 2. 127I
nqcc's in tg-3-Iodo-1,1,1-trifluorobutane
(MHz). |
|
|
|
|
|
|
|
|
|
|
|
Calc (1) was made on the
MP2/6-311+G(2d,2p) optimized molecular structure. |
|
|
Calc (2) was made on this same
structure but with empirically corrected C-C, CF, and CH bond lengths. |
|
|
|
|
|
|
|
|
|
|
|
|
|
Calc (1) |
|
Calc (2) |
|
Expt [1] |
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
- |
1488.1 |
- |
1486.4 |
- |
1473.890(40) |
|
|
Xbb |
|
675.7 |
|
675.1 |
|
656.689(46) * |
|
|
Xcc |
|
812.4 |
|
811.3 |
|
817.2013(236) |
|
|
Xab |
|
766.6 |
|
766.8 |
|
801.934(44) ** |
|
|
Xac |
|
454.8 |
|
455.2 |
|
445.207(67) ** |
|
|
Xbc |
|
- 144.7 |
|
- 144.5 |
|
- 149.799(35) ** |
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
14.0 (1.42 %)
|
|
13.3 (1.36 %)
|
|
|
|
|
RSD |
|
15.2 (1.23 %) |
|
15.2 (1.23 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
898.9 |
|
898.1 |
|
900.662(33) |
|
|
Xyy |
|
919.8 |
|
919.5 |
|
924.826(50) |
|
|
Xzz |
- |
1818.7 |
- |
1817.6 |
- |
1825.488(49) |
|
|
ETA |
|
0.01148 |
|
0.01180 |
|
0.01324(3) |
|
|
Øz,CI |
|
0.50 |
|
0.51 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 3. 127I
nqcc's in tg-3-Iodo-1,1,1-trifluorobutane
(MHz). |
|
|
|
|
|
|
|
|
|
|
|
Calc (1) was made on the
MP2/6-311+G(3d,3p) optimized molecular structure. |
|
|
Calc (2) was made on this same
structure but with empirically corrected C-C, CF, and CH bond lengths. |
|
|
|
|
|
|
|
|
|
|
|
|
|
Calc (1) |
|
Calc (2) |
|
Expt [1] |
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
- |
1486.8 |
- |
1484.2 |
- |
1473.890(40) |
|
|
Xbb |
|
675.7 |
|
674.9 |
|
656.689(46) * |
|
|
Xcc |
|
811.1 |
|
809.3 |
|
817.2013(236) |
|
|
Xab |
|
771.5 |
|
771.0 |
|
801.934(44) ** |
|
|
Xac |
|
461.4 |
|
461.8 |
|
445.207(67) ** |
|
|
Xbc |
|
- 147.7 |
|
- 809.3 |
|
- 149.799(35) ** |
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
13.7 (1.40 %)
|
|
12.9 (1.31 %)
|
|
|
|
|
RSD |
|
15.2 (1.23 %) |
|
15.2 (1.23 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
900.2 |
|
898.6 |
|
900.662(33) |
|
|
Xyy |
|
922.7 |
|
922.0 |
|
924.826(50) |
|
|
Xzz |
- |
1822.9 |
- |
1820.6 |
- |
1825.488(49) |
|
|
ETA |
|
0.01238 |
|
0.01282 |
|
0.01324(3) |
|
|
Øz,CI |
|
0.59 |
|
0.61 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 4. 127I
nqcc's in tg-3-Iodo-1,1,1-trifluorobutane
(MHz). |
|
|
|
|
|
|
|
|
|
|
|
Calc (1) was made on the
MP2/6-311+G(df,pd) optimized molecular structure. |
|
|
Calc (2) was made on this same
structure but with empirically corrected C-C, CF, and CH bond lengths. |
|
|
|
|
|
|
|
|
|
|
|
|
|
Calc (1) |
|
Calc (2) |
|
Expt [1] |
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
- |
1478.1 |
- |
1477.1 |
- |
1473.890(40) |
|
|
Xbb |
|
668.4 |
|
668.7 |
|
656.689(46) * |
|
|
Xcc |
|
809.7 |
|
808.4 |
|
817.2013(236) |
|
|
Xab |
|
778.3 |
|
777.0 |
|
801.934(44) ** |
|
|
Xac |
|
457.0 |
|
457.3 |
|
445.207(67) ** |
|
|
Xbc |
|
- 147.8 |
|
- 147.5 |
|
- 149.799(35) ** |
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
8.4 (0.85 %)
|
|
8.8 (0.89 %)
|
|
|
|
|
RSD |
|
15.2 (1.23 %) |
|
15.2 (1.23 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
897.4 |
|
896.4 |
|
900.662(33) |
|
|
Xyy |
|
920.9 |
|
920.5 |
|
924.826(50) |
|
|
Xzz |
- |
1818.3 |
- |
1816.9 |
- |
1825.488(49) |
|
|
ETA |
|
0.01288 |
|
0.01331 |
|
0.01324(3) |
|
|
Øz,CI |
|
0.58 |
|
0.60 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Table 5. tg-3-Iodo-1,1,1-trifluorobutane.
Selected structure
parameters (Å and
degrees). Complete structures are given here in Z-matrix representation. |
| |
|
|
|
|
r (1) = MP2/6-311+G(d,p) opt |
|
r (2) = r (1) with
corrected C-C, CF, and CH bond lengths. |
|
|
|
|
| Point Group C1 |
|
r (1) |
r (2) |
|
|
|
|
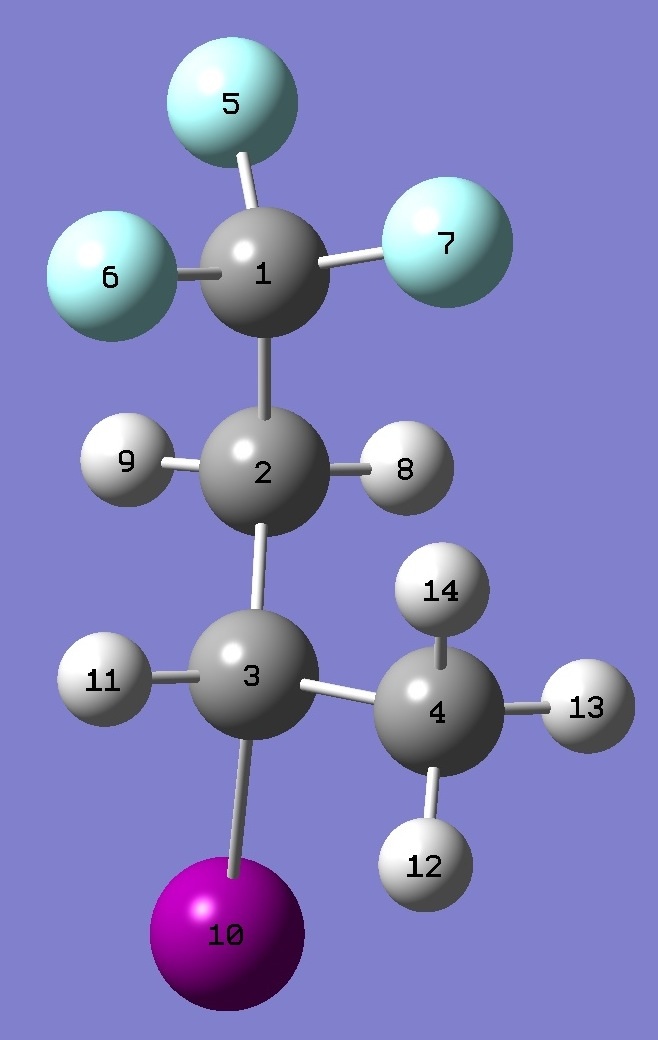
|
C(1)C(2) |
1.5120 |
1.5051 |
| C(2)C(3) |
1.5295 |
1.5215 |
| C(3)C(4) |
1.5234 |
1.5158 |
| C(3)I |
2.1826 |
2.1826 |
| C(1)C(2)C(3) |
113.83 |
113.83 |
| C(2)C(3)C(4) |
115.11 |
115.11 |
| C(2)C(3)I |
107.49 |
107.49 |
| C(1)C(2)C(3)C(4) |
- 63.19 |
- 63.19 |
| C(1)C(2)C(3)I |
174.29 |
174.29 |
| |
|
|
| r (1) = MP2/6-311+G(2d,2p) opt |
| r (2) = r (1) with
corrected C-C, CF, and CH bond lengths. |
|
|
|
|
r (1) |
r (2) |
|
|
|
| C(1)C(2) |
1.5076 |
1.5051 |
| C(2)C(3) |
1.5230 |
1.5215 |
| C(3)C(4) |
1.5179 |
1.5158 |
| C(3)I |
2.1557 |
2.1557 |
| C(1)C(2)C(3) |
113.55 |
113.55 |
| C(2)C(3)C(4) |
115.00 |
115.00 |
| C(2)C(3)I |
107.72 |
107.72 |
| C(1)C(2)C(3)C(4) |
- 60.91 |
- 60.91 |
| C(1)C(2)C(3)I |
176.04 |
176.04 |
| |
|
|
|
r (1) = MP2/6-311+G(3d,3p) opt |
|
r (2) = r (1) with
corrected C-C, CF, and CH bond lengths. |
|
|
|
|
|
|
r (1) |
r (2) |
|
|
|
|
|
C(1)C(2) |
1.5098 |
1.5051 |
|
C(2)C(3) |
1.5246 |
1.5215 |
|
C(3)C(4) |
1.5188 |
1.5158 |
|
C(3)I |
2.1712 |
2.1712 |
|
C(1)C(2)C(3) |
113.31 |
113.31 |
|
C(2)C(3)C(4) |
114.88 |
114.88 |
|
C(2)C(3)I |
107.30 |
107.30 |
|
C(1)C(2)C(3)C(4) |
- 62.58 |
- 62.58 |
|
C(1)C(2)C(3)I |
175.33 |
175.33 |
|
|
|
|
|
r (1) = MP2/6-311+G(df,pd) opt |
|
r (2) = r (1) with
corrected C-C, CF, and CH bond lengths. |
|
|
|
|
|
|
r (1) |
r (2) |
|
|
|
|
|
C(1)C(2) |
1.5130 |
1.5051 |
|
C(2)C(3) |
1.5256 |
1.5215 |
|
C(3)C(4) |
1.5199 |
1.5158 |
|
C(3)I |
2.1529 |
2.1529 |
|
C(1)C(2)C(3) |
113.92 |
113.92 |
|
C(2)C(3)C(4) |
114.94 |
114.94 |
|
C(2)C(3)I |
107.62 |
107.62 |
|
C(1)C(2)C(3)C(4) |
- 62.27 |
- 62.27 |
|
|
C(1)C(2)C(3)I |
175.08 |
175.08 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Table 6.
tg-3-Iodo-1,1,1-trifluorobutane.
Rotational constants
(MHz). |
| |
|
|
|
|
|
r (1) = MP2/6-311+G(d,p) opt |
|
r (2) = r (1) with
corrected C-C, CF, and CH bond lengths. |
|
|
|
|
|
|
|
r (1) |
r (2) |
Expt [1] |
|
|
|
|
|
|
A |
2941.4 |
2969.9 |
2930.12759(98) |
|
B |
446.2 |
448.7 |
449.276659(138) |
|
C |
420.9 |
423.4 |
423.278388(300) |
| |
|
|
|
|
|
r (1) = MP2/6-311+G(2d,2p) opt |
|
r (2) = r (1) with
corrected C-C, CF, and CH bond lengths. |
|
|
|
|
|
|
|
r (1) |
r (2) |
Expt [1] |
|
|
|
|
|
|
A |
2978.8 |
2998.6 |
2930.12759(98) |
|
B |
451.6 |
452.7 |
449.276659(138) |
|
C |
426.4 |
427.5 |
423.278388(300) |
| |
|
|
|
|
|
r (1) = MP2/6-311+G(3d,3p) opt |
|
r (2) = r (1) with
corrected C-C, CF, and CH bond lengths. |
|
|
|
|
|
|
|
r (1) |
r (2) |
Expt [1] |
|
|
|
|
|
|
A |
2965.4 |
2989.0 |
2930.12759(98) |
|
B |
449.6 |
451.4 |
449.276659(138) |
|
C |
424.5 |
426.2 |
423.278388(300) |
| |
|
|
|
|
|
r (1) = MP2/6-311+G(df,pd) opt |
|
r (2) = r (1) with
corrected C-C, CF, and CH bond lengths. |
|
|
|
|
|
|
|
r (1) |
r (2) |
Expt [1] |
|
|
|
|
|
|
A |
2984.1 |
2982.7 |
2930.12759(98) |
|
B |
452.2 |
453.6 |
449.276659(138) |
|
C |
426.7 |
428.1 |
423.278388(300) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[1]
S.A.Cooke, private communication. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
t-CH3CH2CH2I |
g-CH3CH2CH2I |
CH3I |
CH3CH2I |
|
|
g-CH2ICH2F |
CH3-O-CH2I |
CH3CHICH3 |
(CH3)2CHCH2I |
|
|
t-CF3CF2CH2I |
t-CF3CF2CF2I |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Iodine |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CF3CH2CHICH3.html |
|
|
|
|
|
|
Last
Modified 1 March 2011 |
|
|
|
|
|
|
|
|
|
|

