|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C3H4N2O2 |
|
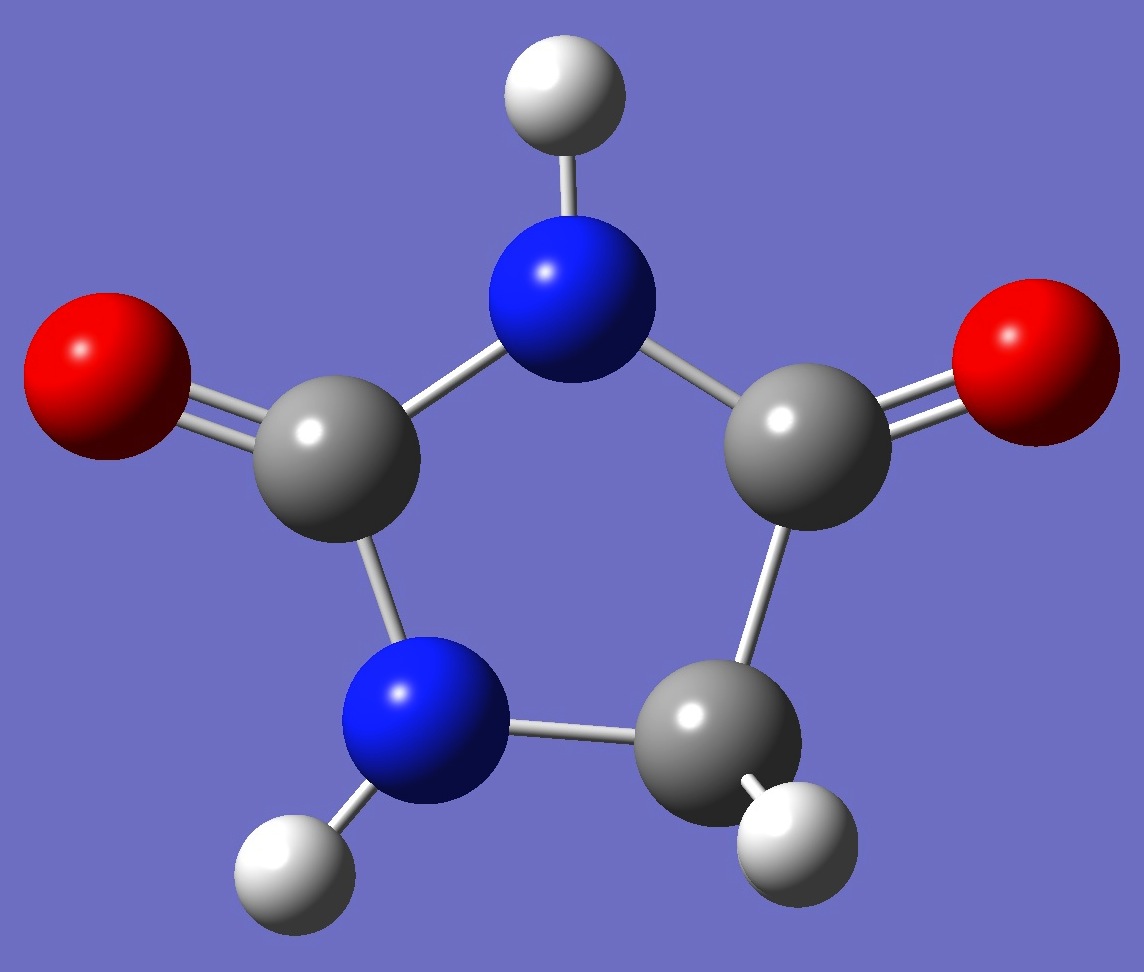
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nitrogen
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
in Imidazolidine-2,4-dione (Hydantoin)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nitrogen nqcc's in hydantoin were determined by Alonso, et al. [1].
|
|
|
|
|
|
|
|
|
|
|
|
|
Calculation of the
nitrogen nqcc tensors in hydantoin was made here on an ropt structure given by B3LYP/cc-pVTZ optimization. These calculated nqcc's are compared with the experimental values in
Tables 1 and 2. Structure parameters are given in Z-matrix format in Table 3, rotational constants in Table 4.
|
|
|
|
|
|
|
|
|
|
|
|
|
Calculation of the efg's/nqcc's was made with both B3PW91/6-311+G(df,pd) and B3PW91/6-311+G(d,p) models, the latter shown to perform better than the former for modeling conjugated pi-electron systems [2].
|
|
|
|
|
|
|
|
|
|
|
|
|
In Tables 1 and 2, subscripts a,b,c
refer to the
principal axes of the inertia tensor; x,y,z to the principal axes
of the nqcc tensor. ETA = (Xxx - Xyy)/Xzz. RMS is the root mean square
difference between calculated and experimental diagonal nqcc's
(percentage of the average of the magnitudes of the experimental
nqcc's). RSD is the calibration residual standard deviation of the model for calculation of nitrogen efg's/nqcc's. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 1. 14N(1) nqcc's in Hydantoin
(MHz). Calculation was made on the ropt molecular structure with (1) B3PW91/6-311+G(df,pd) and (2) B3PW91/6-311+G(d,p) models.
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc (1)
|
|
Calc (2)
|
|
Expt [1]
|
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
|
2.655
|
|
2.722
|
|
2.5900(41)
|
|
|
Xbb |
|
2.225
|
|
2.322
|
|
2.1438(70)
|
|
|
Xcc |
-
|
4.880
|
-
|
5.044
|
-
|
4.7338(70)
|
|
|
|Xab| |
|
0.207
|
|
0.204
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RMS
|
|
0.104 (3.3 %)
|
|
0.220 (7.0%)
|
|
|
|
|
RSD |
|
0.030 (1.3 %) |
|
0.086 (3.8 %) |
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
2.738
|
|
2.808
|
|
|
|
|
Xyy |
|
2.142
|
|
2.236
|
|
|
|
|
Xzz |
-
|
4.880 |
-
|
5.044
|
|
|
|
|
ETA |
-
|
0.122
|
-
|
0.113
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 2. 14N(3) nqcc's in Hydantoin
(MHz). Calculation was made on the ropt molecular structure with (1) B3PW91/6-311+G(df,pd) and (2) B3PW91/6-311+G(d,p) models.
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc (1)
|
|
Calc (2)
|
|
Expt [1]
|
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
|
1.607
|
|
1.679
|
|
1.6315(51)
|
|
|
Xbb |
|
1.779
|
|
1.858
|
|
1.8321(75)
|
|
|
Xcc |
-
|
3.386
|
-
|
3.536
|
-
|
3.4635(75)
|
|
|
|Xab| |
|
0.076
|
|
0.073
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RMS
|
|
0.056 (2.4 %)
|
|
0.052 (2.3 %)
|
|
|
|
|
RSD |
|
0.030 (1.3 %) |
|
0.086 (3.8 %) |
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
1.578
|
|
1.653
|
|
|
|
|
Xyy |
|
1.808
|
|
1.883
|
|
|
|
|
Xzz |
-
|
3.386
|
-
|
3.536
|
|
|
|
|
ETA |
|
0.0679
|
|
0.0653
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
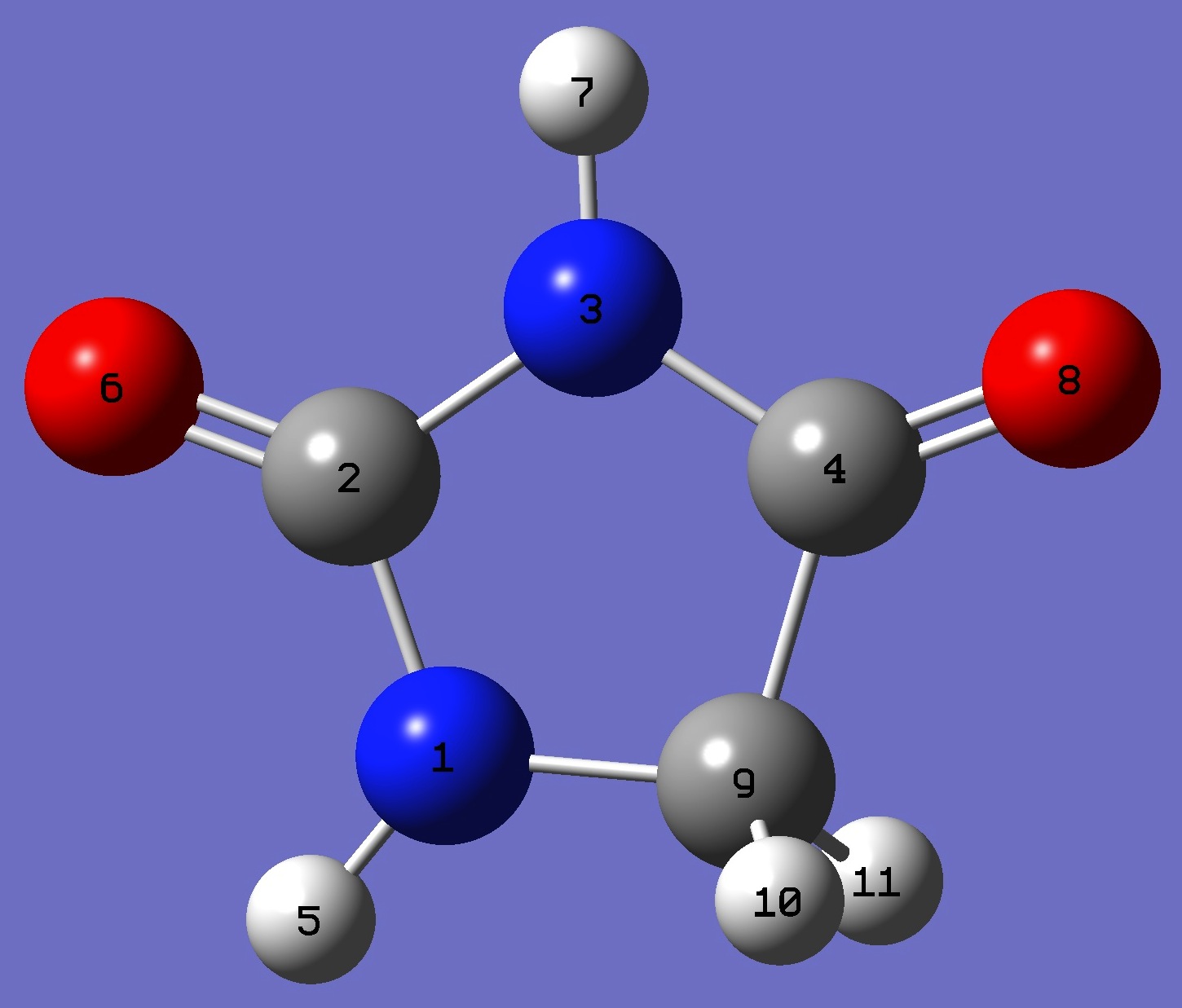
Table 3. Hydantoin. Structure parameters, ropt = B3LYP/cc-pVTZ (┼ and
degrees).
|
| |
|
|
|
|
|
| 
|
|
N
C,1,B1
N,2,B2,1,A1
C,3,B3,2,A2,1,D1,0
H,1,B4,2,A3,3,D2,0
O,2,B5,1,A4,4,D3,0
H,3,B6,2,A5,1,D4,0
O,4,B7,3,A6,2,D5,0
C,1,B8,2,A7,6,D6,0
H,9,B9,1,A8,2,D7,0
H,9,B10,1,A9,2,D8,0
|
|
|
|
|
|
|
|
|
|
B1=1.3665913
B2=1.4094302
B3=1.3750941
B4=1.00330429
B5=1.20586036
B6=1.00648988
B7=1.20427321
B8=1.44738042
B9=1.09197362
B10=1.09197362
A1=105.28084324
A2=113.68550985
A3=120.80025879
A4=128.71325661
A5=122.0769678
A6=127.54919904
A7=113.35514827
A8=113.36904371
A9=113.36904371
D1=0.
D2=180.
D3=180.
D4=180.
D5=180.
D6=180.
D7=117.89506202
D8=-117.89506202
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table 4. Hydantoin. Rotational Constants (MHz).
|
|
|
|
|
|
|
ropt |
Expt [1]
|
|
|
|
|
|
A
|
6568.
|
6537.73799(86)
|
|
B
|
2287.
|
2291.37278(16)
|
|
C
|
1715.
|
1716.47204(22)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[1] E.R.Alonso, L.Kolesnikovß, and J.L.Alonso, J.Chem.Phys. 147,124312(2017).
|
|
|
[2] R.Kannengie▀er, W.Stahl, H.V.L.Nguyen, and W.C.Bailey, J.Mol.Spectrosc. 317,50(2015).
|
|
|
|
|
|
|
|
|
|
|
|
|
H.Ozeki, R.Miyahara, H.Ihara, S.Todaka, K.Kobayashi, and M.Ohishi, Abstract TH10,
71st International Symposium on Molecular Spectroscopy,
Champaign-Urbana, Ill 2016.
|
|
|
Ibid, A&A 600,A44(2017).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Imidazole
|
Uracil
|
Thymine |
2-Pyridone |
|
N-Vinylformamide
|
N-Methyldiacetamide
|
|
Maleimide
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Nitrogen |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Hydantoin.html |
|
|
|
|
|
|
Last
Modified 29 Sept 2017 |
|
|
|
|
|
|
|
|
|
|

