|
| |
|
|
|
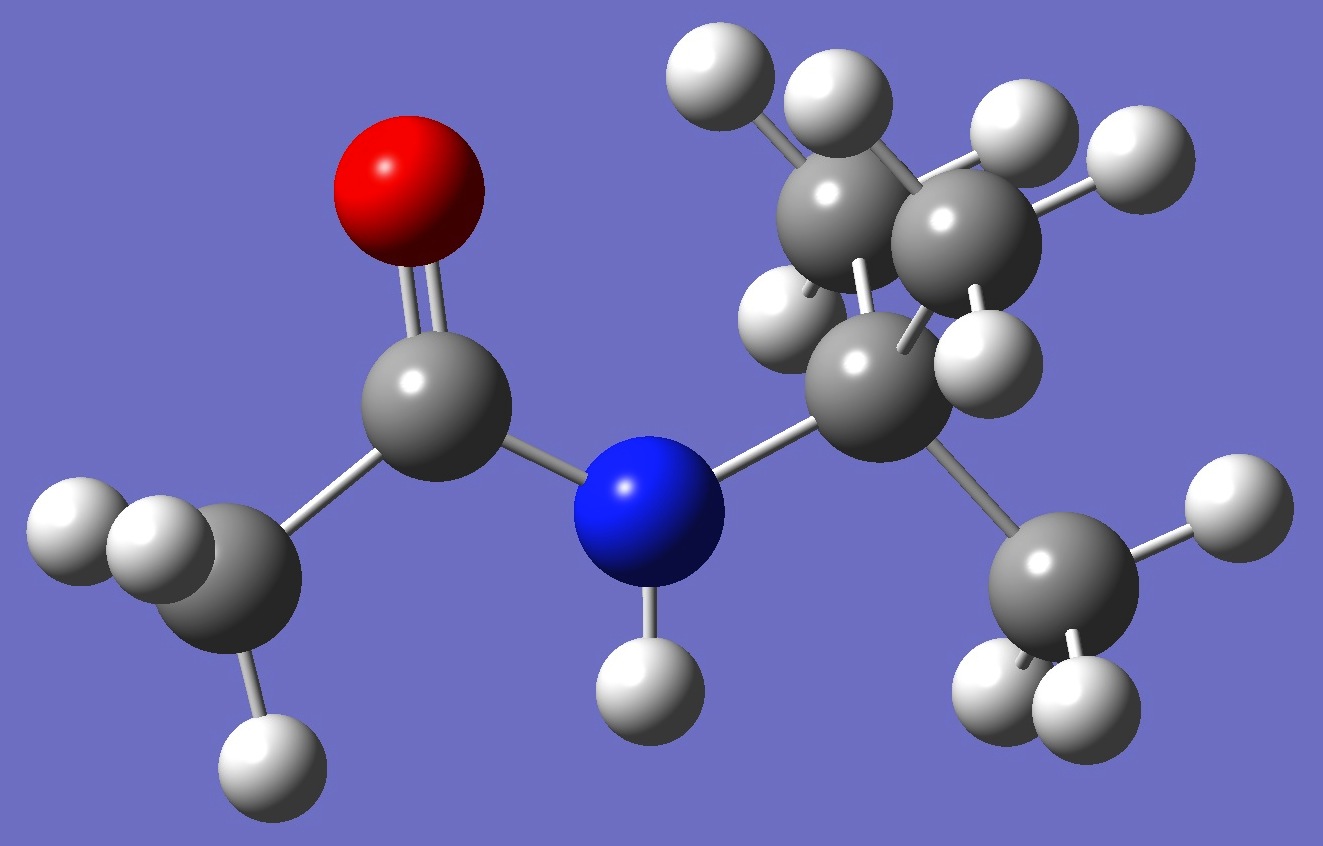
Table 2. N-tert-Butylacetamide. ropt(1) = HF/6-311++G(d,p) and ropt(2) = HF/6-311++G(3df,3pd) optimized structure parameters (Å and
degrees).
|
| |
|
|
|
|
|
|
|
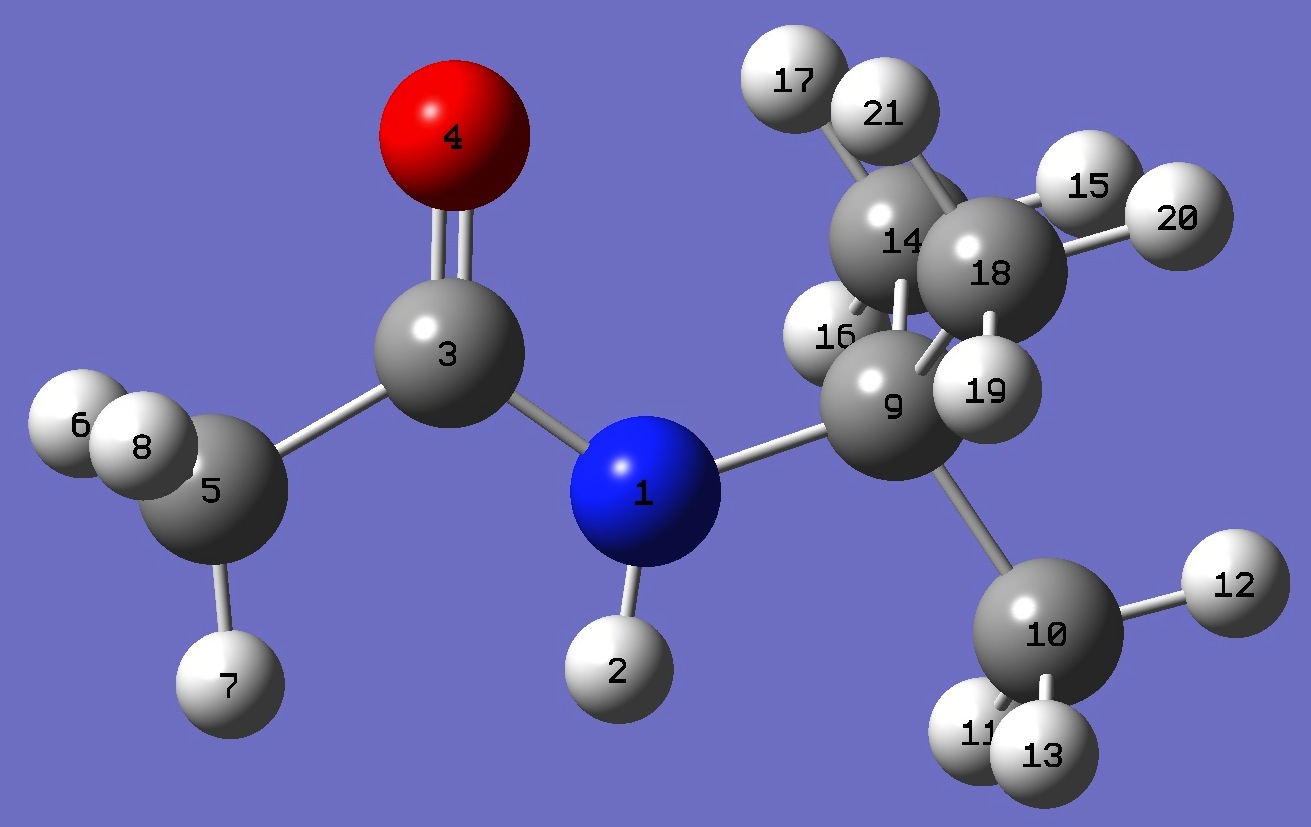
|
N
H,1,B1
C,1,B2,2,A1
O,3,B3,1,A2,2,D1,0
C,3,B4,1,A3,4,D2,0
H,5,B5,3,A4,1,D3,0
H,5,B6,3,A5,1,D4,0
H,5,B7,3,A6,1,D5,0
C,1,B8,3,A7,4,D6,0
C,9,B9,1,A8,3,D7,0
H,10,B10,9,A9,1,D8,0
H,10,B11,9,A10,1,D9,0
H,10,B12,9,A11,1,D10,0
C,9,B13,1,A12,3,D11,0
H,14,B14,9,A13,1,D12,0
H,14,B15,9,A14,1,D13,0
H,14,B16,9,A15,1,D14,0
C,9,B17,1,A16,3,D15,0
H,18,B18,9,A17,1,D16,0
H,18,B19,9,A18,1,D17,0
H,18,B20,9,A19,1,D18,0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ropt(1) |
ropt(2) |
|
|
|
|
B1=0.99166682
B2=1.35117745
B3=1.19928341
B4=1.5155969
B5=1.08374172
B6=1.0839508
B7=1.08374172
B8=1.47375846
B9=1.53179397
B10=1.08713904
B11=1.08437831
B12=1.08713904
B13=1.53377988
B14=1.08654712
B15=1.08639978
B16=1.08147639
B17=1.53377988
B18=1.08639978
B19=1.08654712
B20=1.08147639
A1=116.72408965
A2=123.8474427
A3=115.63308723
A4=108.29170471
A5=113.80910699
A6=108.29170471
A7=126.58119604
A8=106.02231262
A9=111.11702252
A10=110.20563029
A11=111.11702252
A12=110.4459299
A13=109.9243619
A14=110.51814132
A15=111.00387703
A16=110.4459299
A17=110.51814132
A18=109.9243619
A19=111.00387703
D1=180.
D2=180.
D3=-121.68721051
D4=0.
D5=121.68721051
D6=0.
D7=180.
D8=60.47978483
D9=180.
D10=-60.47978483
D11=-61.34764484
D12=-177.30177352
D13=-57.97419947
D14=62.58239806
D15=61.34764484
D16=57.97419947
D17=177.30177352
D18=-62.58239806
|
B1=0.98920197
B2=1.3475814
B3=1.19663021
B4=1.51429528
B5=1.08128262
B6=1.08140878
B7=1.08128262
B8=1.47028305
B9=1.52944027
B10=1.08462152
B11=1.08170512
B12=1.08462152
B13=1.53153757
B14=1.08385354
B15=1.083891
B16=1.07889603
B17=1.53153757
B18=1.083891
B19=1.08385354
B20=1.07889603
A1=116.71450471
A2=123.86361
A3=115.67987532
A4=108.25655891
A5=113.83010151
A6=108.25655891
A7=126.63985668
A8=106.05888879
A9=111.0768953
A10=110.20284832
A11=111.0768953
A12=110.50534888
A13=109.91611051
A14=110.51581369
A15=110.96209458
A16=110.50534888
A17=110.51581369
A18=109.91611051
A19=110.96209458
D1=180.
D2=180.
D3=-121.70302441
D4=0.
D5=121.70302441
D6=0.
D7=180.
D8=60.44335989
D9=180.
D10=-60.44335989
D11=-61.34346157
D12=-177.32225319
D13=-57.98133798
D14=62.53753479
D15=61.34346157
D16=57.98133798
D17=177.32225319
D18=-62.53753479
|
|
|
|
|
|
|
|