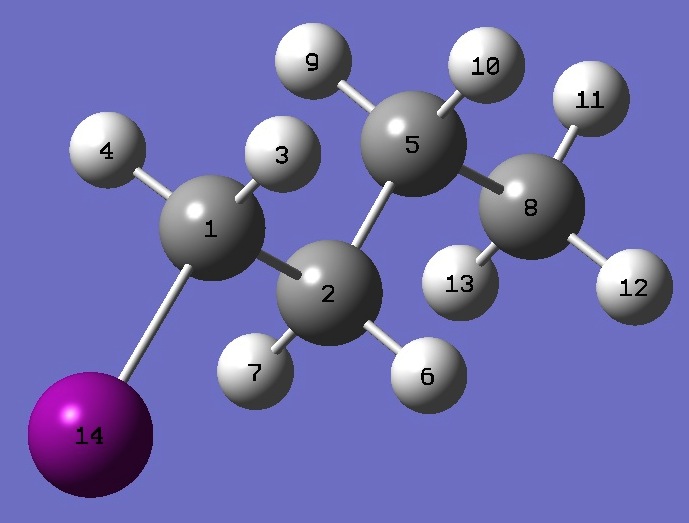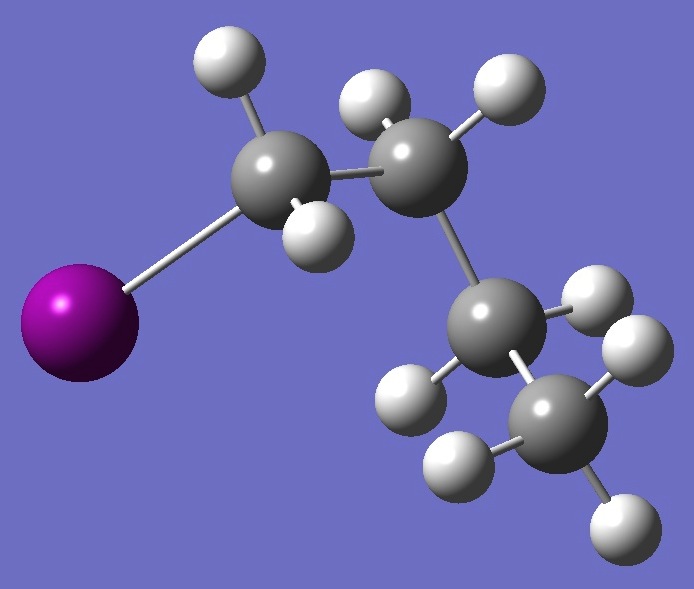
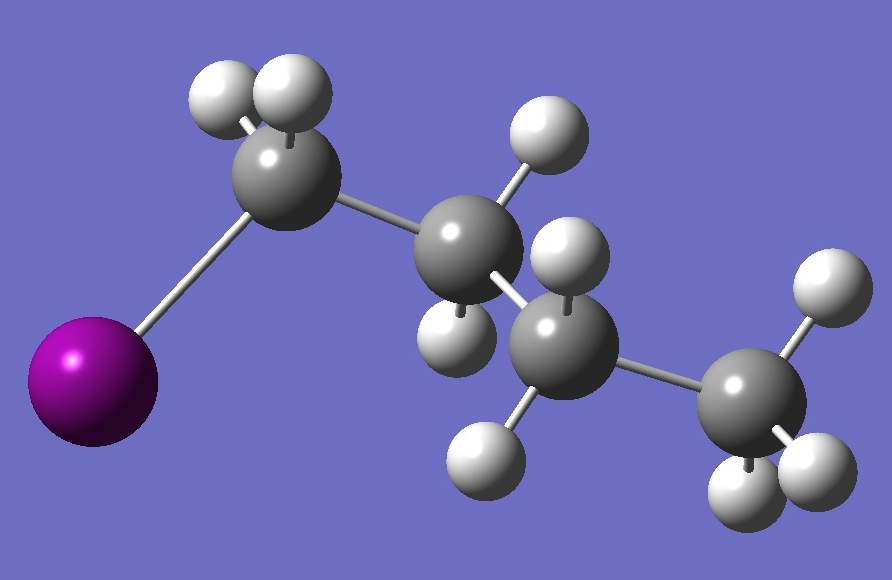
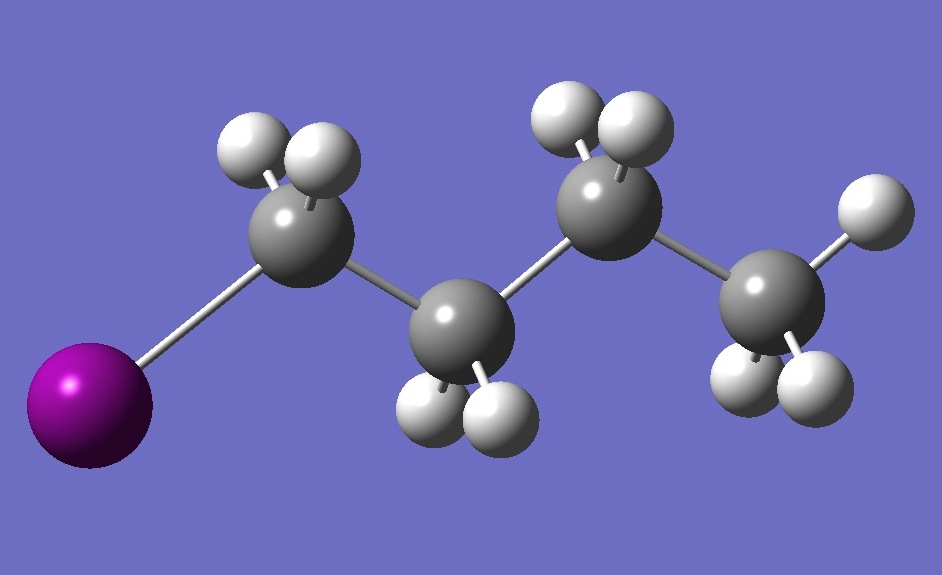
| |
||||||||
| Table 1. 127I nqcc's in AA-1-Iodobutane (MHz). Calculation was made on the following structures: ropt (1) = MP2/6-311+G(d,p) optimization, and ropt (2) = MP2/6-311+G(3df,3pd) optimization. | ||||||||
| Calc. (1) |
Calc. (2) | Expt [1] | ||||||
| |
||||||||
| Xaa | - |
1316.7 |
- |
1286.3 |
- |
1294.041(34) |
||
| Xbb | 399.5 |
382.6 |
380.05(6) |
|||||
| Xcc | 917.2 |
903.7 |
913.99(7) |
|||||
| |Xab| | 1062.4 |
1056.8 |
1070.28(6) |
|||||
| RMS | 17.4 (2.01 %) |
7.6 (0.88 %) |
||||||
| RSD | 15.2 (1.23 %) | 15.2 (1.23 %) | ||||||
| Xxx | 907.1 |
894.7 |
901.73(7) |
|||||
| Xyy | 917.2 | 903.7 |
913.99(7) | |||||
| Xzz | - |
1824.2 |
- |
1798.4 |
- |
1815.72(6) |
||
| ETA | 0.0055 |
0.0050 |
0.00675(6) |
|||||
| Øz,a | 25.53 |
25.85 |
||||||
| Øa,CI | 24.80 |
25.01 |
||||||
| Øz,CI | 0.73 |
0.84 |
||||||
| Table 3. AA-1-Iodobutane. Rotational Constants (MHz). ropt (1) = MP2/6-311+G(d,p), and ropt (2) = MP2/6-311+G(3df,3pd) optimization. | ||||
| ropt(1) | ropt(2) | Expt [1] | ||
| A | 15187. |
15310. |
15052.043(9) |
|
| B | 727, |
740. |
732.93002(18) |
|
| C | 706. |
719. |
711.34567(20) |
|
| Table 4. AA-1-Iodobutane. Quartic Centrifugal Distortion Constants (kHz). Calc = B3LYP/6-311+G(d,p). |
||||||
| Calc | Expt [1] | |||||
| D_J |
0.0411 |
0.0459(11) |
||||
| D_JK |
- |
2.57 |
- |
2.867(17) |
||
| D_K |
164. |
|||||
| d_1 |
- |
0.00264 |
||||
| d_2 |
- |
0.00003 |
||||