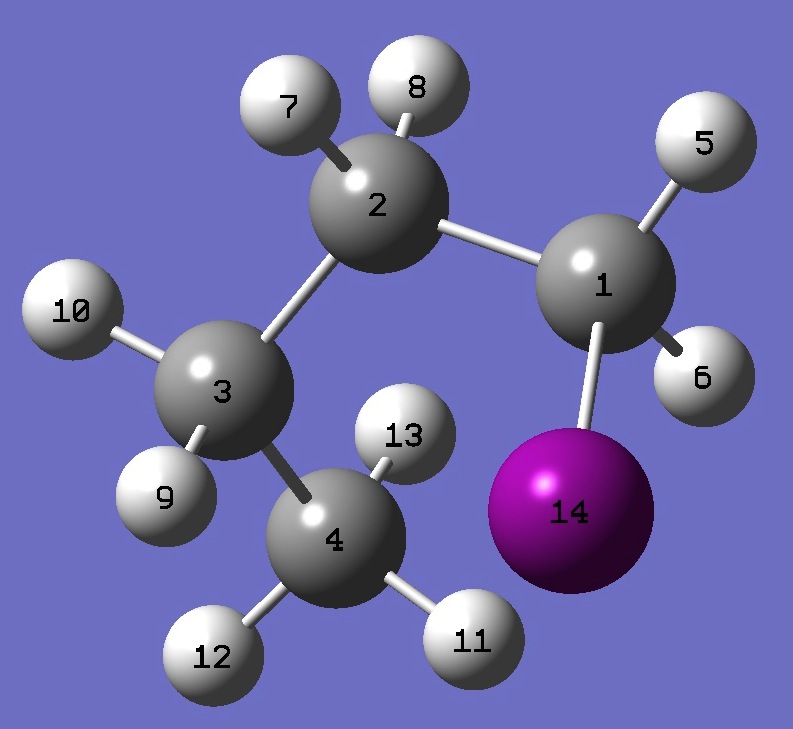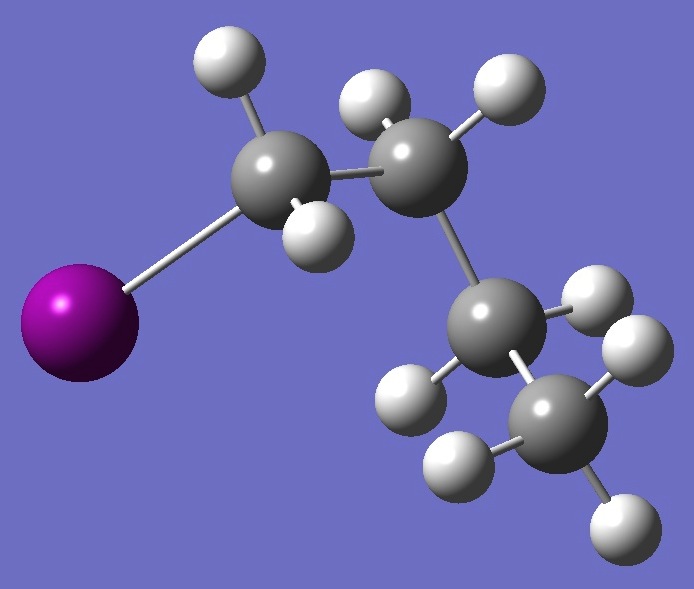
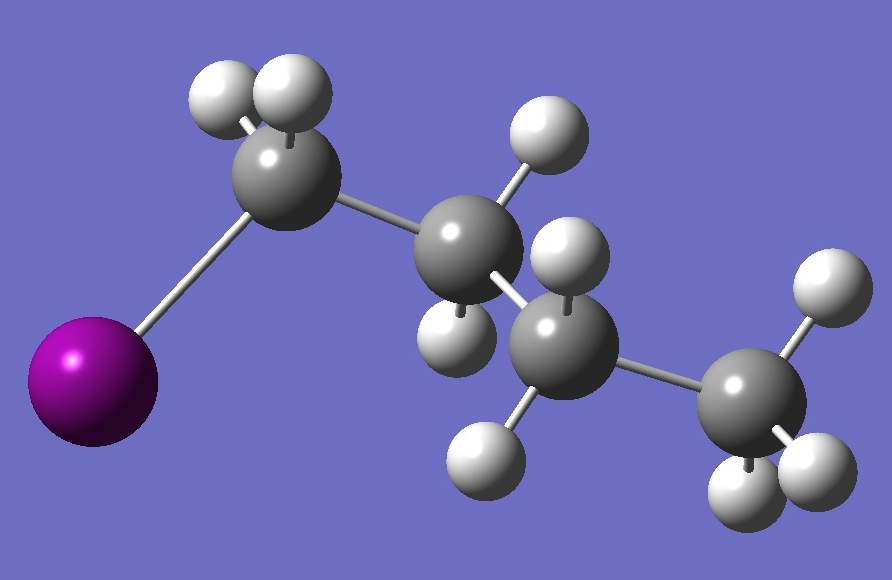
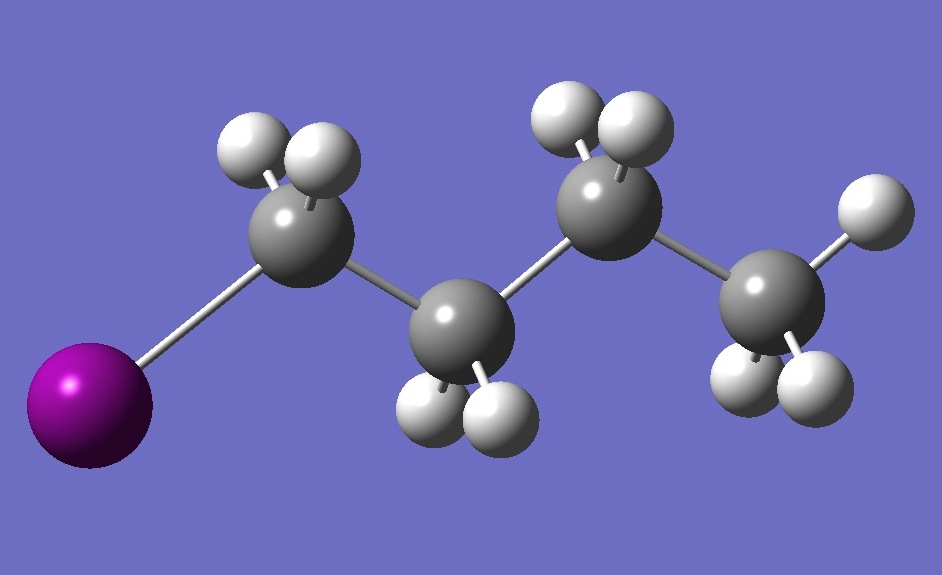
| |
||||||||
| Table 1. 127I nqcc's in GG-1-Iodobutane (MHz). Calculation was made on the following structures: ropt (1) = MP2/6-311+G(d,p) optimization, and ropt (2) = MP2/6-311+G(3df,3pd) optimization. | ||||||||
| |
||||||||
| Calc. (1) |
Calc. (2) | Expt. [1] | ||||||
| |
||||||||
| Xaa | - 756.7 |
- 712.0 |
- 752.593(28) |
|||||
| Xbb | 163.7 |
133.2 |
157.01(5) |
|||||
| Xcc | 593.0 |
578.8 |
595.58(6) |
|||||
| Xab | - |
1119.1 |
- |
1115.4 |
- |
1116.74(13) |
||
| Xac | - 702.1 |
- 693.4 |
- 692.17(19) |
|||||
| Xbc | - 464.1 |
- 468.9 |
- 460.66(12) |
|||||
| RMS | 4.8 (0.96 %) |
28.8 (5.75 %) |
||||||
| RSD | 15.2 (1.23 %) | 15.2 (1.23 %) | ||||||
| Xxx | 887.7 |
874.0 |
576.17(16) |
|||||
| Xyy | 914.6 |
905.3 |
1182.40(19) |
|||||
| Xzz | - |
1802.3 |
- |
1779.3 |
- |
1758.57(14) |
||
| ETA | 0.0149 |
0.0176 |
0.34473(15) |
|||||
| Øz,CI | 0.64 |
0.58 |
||||||
| Table 3. GG-1-Iodobutane. Rotational Constants (MHz). ropt (1) = MP2/6-311+G(d,p) and ropt (2) = MP2/6-311+G(3df,3pd) optimization. | ||||
| ropt(1) | ropt(2) | Expt. [1] | ||
| A | 5831. |
5808. |
5917.262(13) |
|
| B | 1175. |
1221. |
1162.2040(14) |
|
| C | 1092. |
1130. |
1082.7921(12) |
|
| Table 4. GG-1-Iodobutane. Quartic Centrifugal Distortion Constants (kHz). Calc = B3LYP/6-311+G(d,p). |
||||||
| Calc | Expt [1] | |||||
| D_J |
0.614 |
0.816(10) |
||||
| D_JK |
- |
7.19 |
- |
7.18(5) |
||
| D_K |
41.8 |
34.9(38) |
||||
| d_1 |
- |
0.0934 |
- |
0.133(12) |
||
| d_2 |
- |
0.00282 |
||||