|
| |
|
|
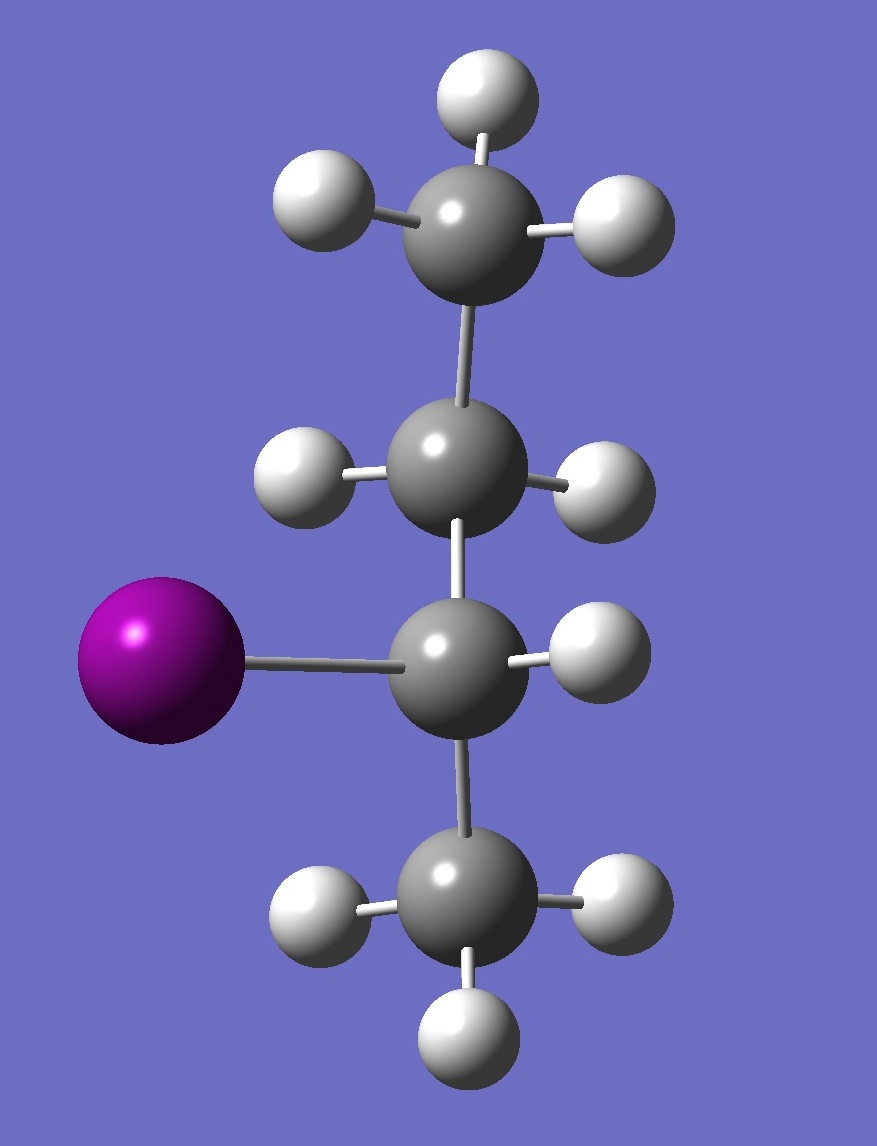
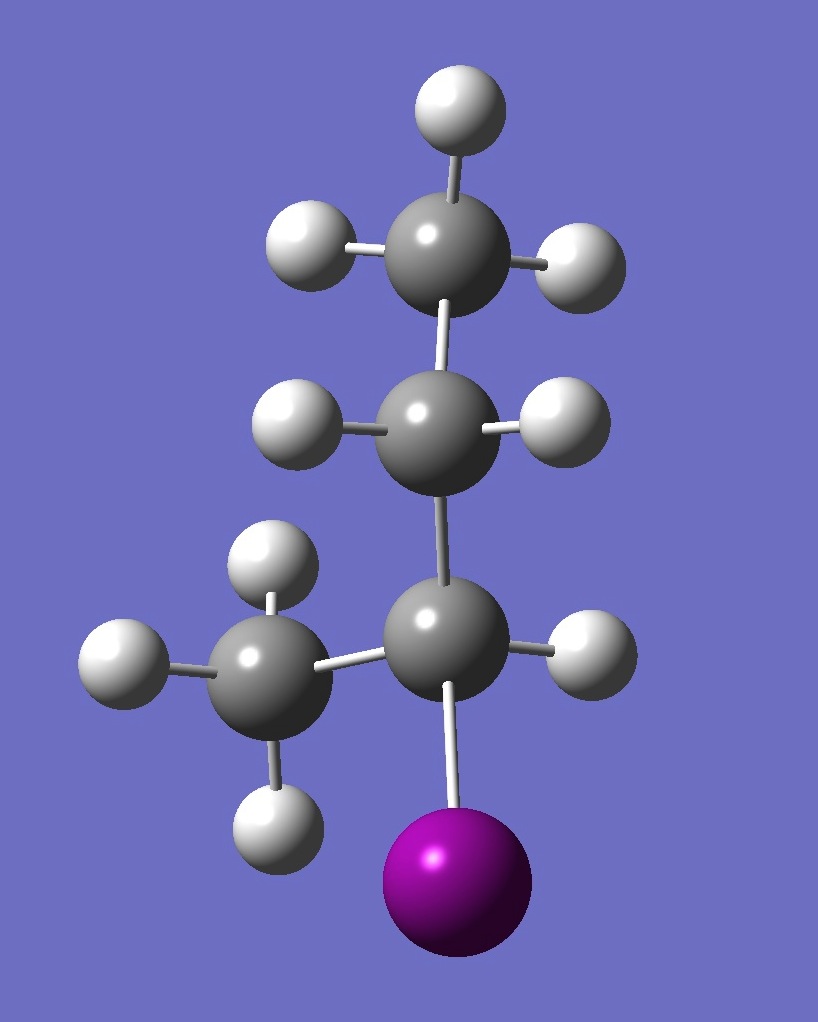
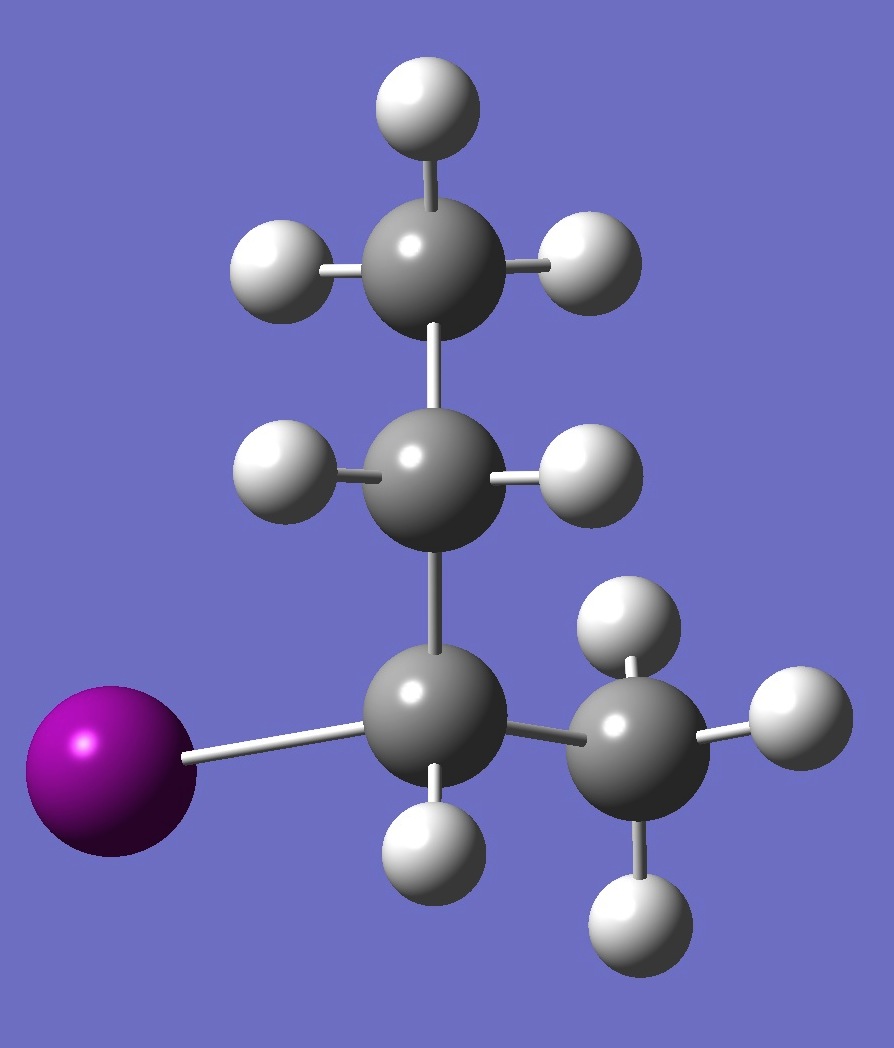
| Table 2. A-2-Iodobutane ropt (1) = MP2/6-311+G(d,p) optimization, and ropt (2) = MP2/6-311+G(3df,3pd) structure parameters
(Å and degrees). |
| |
|
|
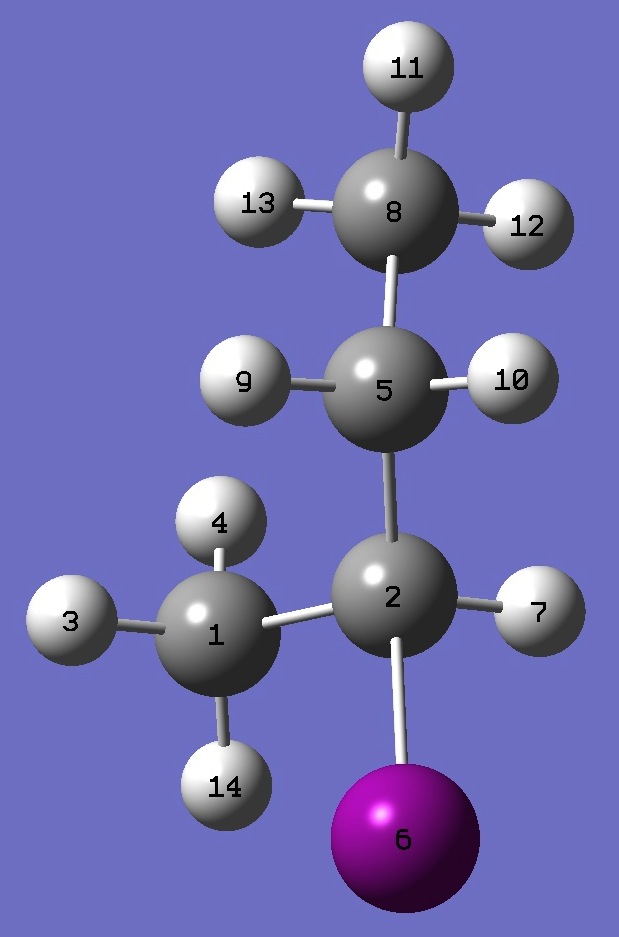
|
C
C,1,B1
H,1,B2,2,A1
H,1,B3,2,A2,3,D1,0
C,2,B4,1,A3,3,D2,0
I,2,B5,1,A4,5,D3,0
H,2,B6,1,A5,5,D4,0
C,5,B7,2,A6,1,D5,0
H,5,B8,2,A7,1,D6,0
H,5,B9,2,A8,1,D7,0
H,8,B10,5,A9,2,D8,0
H,8,B11,5,A10,2,D9,0
H,8,B12,5,A11,2,D10,0
H,1,B13,2,A12,5,D11,0
|
|
|
| ropt (1) |
ropt (2) |
|
|
|
B1=1.52309647
B2=1.09439919
B3=1.09539193
B4=1.52710721
B5=2.18633275
B6=1.0940065
B7=1.53283919
B8=1.09645871
B9=1.09450454
B10=1.09388025
B11=1.09529135
B12=1.09280284
B13=1.09211151
A1=110.59000358
A2=109.63487217
A3=114.10860178
A4=109.38550113
A5=110.41269895
A6=112.13185788
A7=109.14581552
A8=109.04493074
A9=110.07532588
A10=110.85224216
A11=111.94756036
A12=111.42203348
D1=119.73776402
D2=-56.5742852
D3=122.73406089
D4=-124.30070661
D5=-61.9440144
D6=60.37450553
D7=176.81988378
D8=-172.75903904
D9=-53.53019543
D10=67.4990844
D11=-177.22470697
|
B1=1.51780948
B2=1.08987151
B3=1.0904817
B4=1.5217983
B5=2.1531448
B6=1.08953756
B7=1.52656517
B8=1.09198305
B9=1.08998223
B10=1.08874766
B11=1.09042816
B12=1.088049
B13=1.0873434
A1=110.48096854
A2=109.79529439
A3=113.84850095
A4=109.27092778
A5=110.5442137
A6=112.00860522
A7=108.87480613
A8=109.0147075
A9=110.26929846
A10=110.93266339
A11=111.86078219
A12=111.31140027
D1=119.74975035
D2=-56.36310512
D3=122.25921707
D4=-124.42416352
D5=-62.6355935
D6=59.61182873
D7=175.90523498
D8=-173.92179995
D9=-54.54315697
D10=66.30418441
D11=-176.86966055
|
|
|
|
|
|