|
| |
|
|
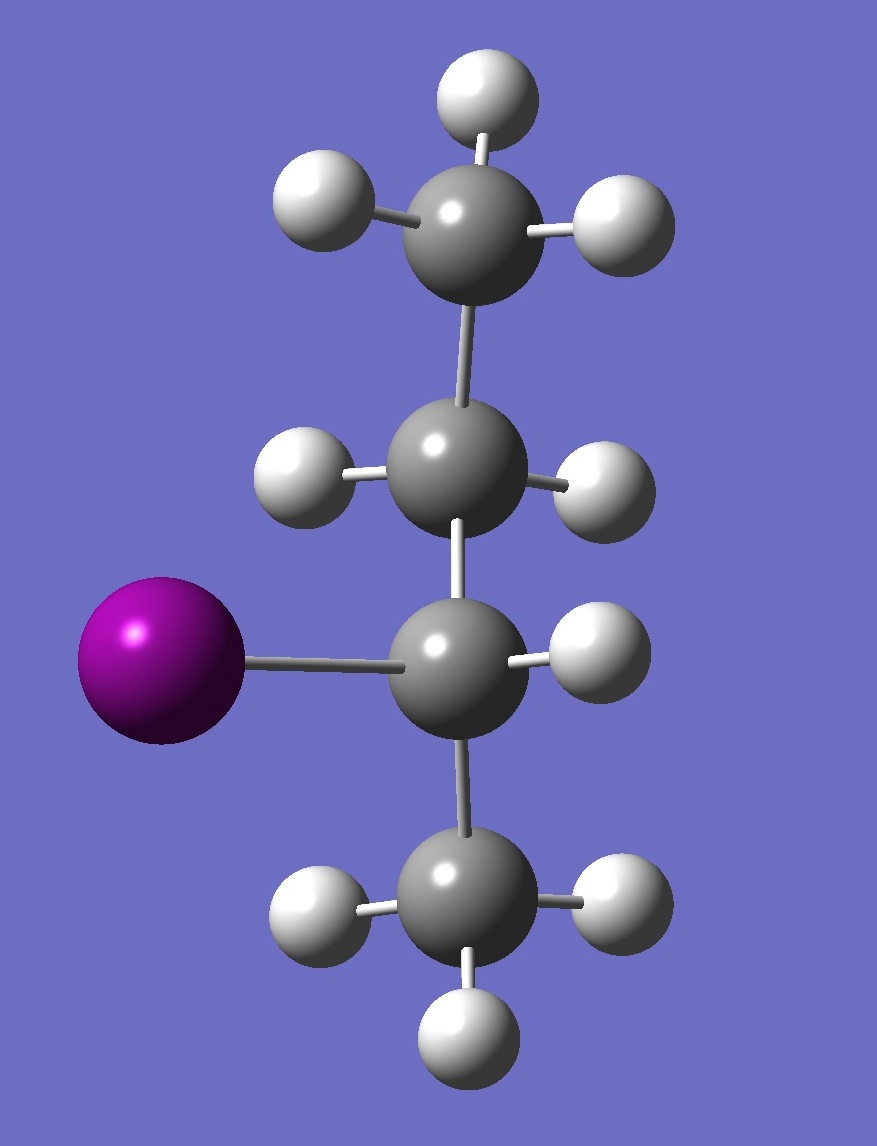
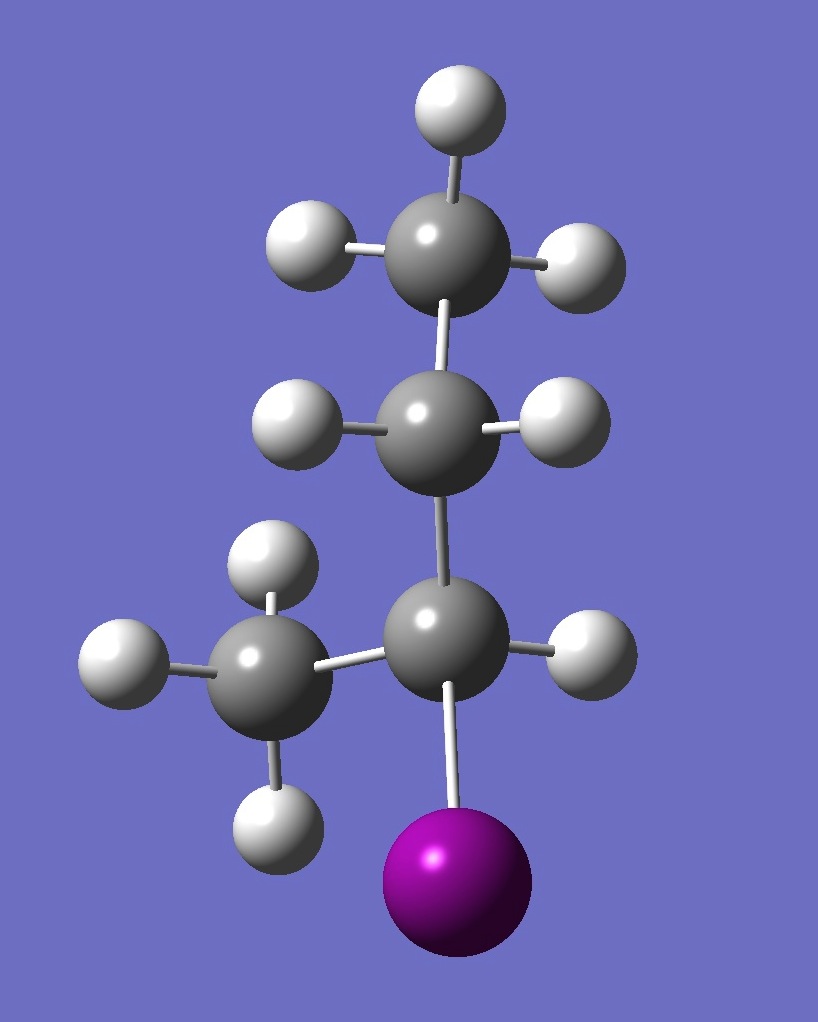
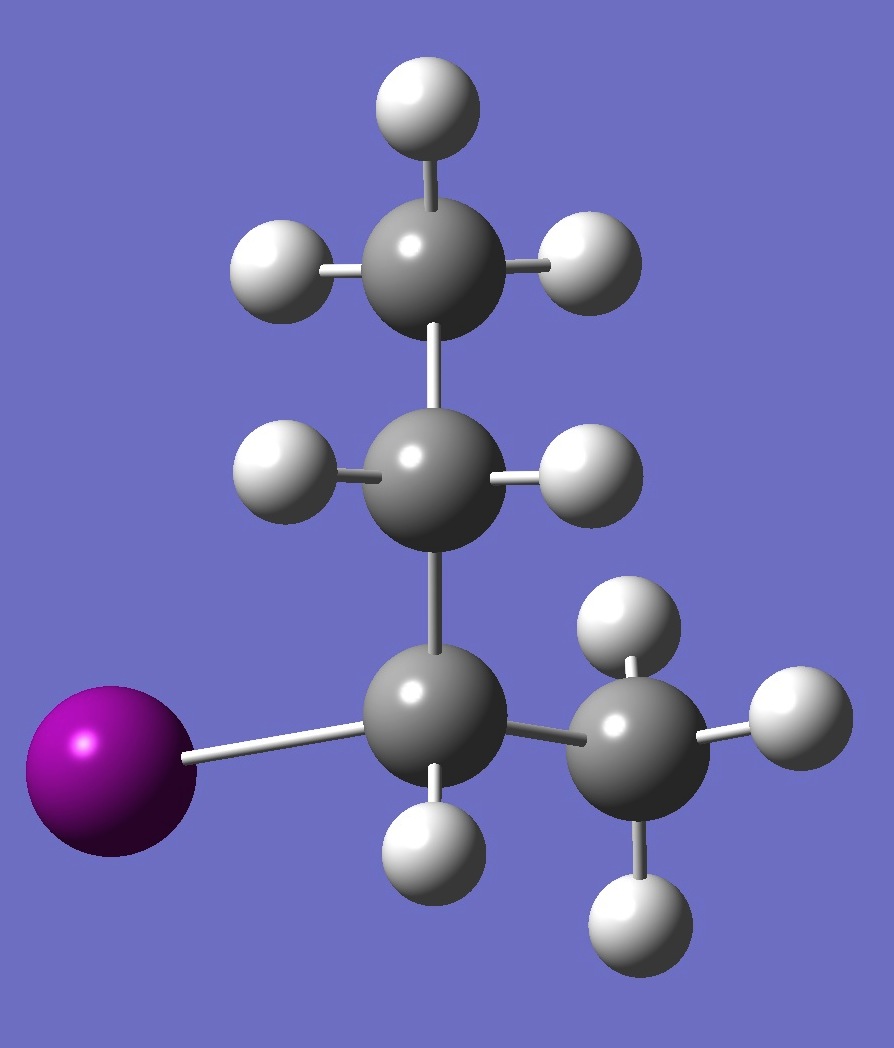
| Table 2. G-2-Iodobutane ropt (1) = MP2/6-311+G(d,p) optimization, and ropt (2) = MP2/6-311+G(3df,3pd) structure parameters
(Å and degrees). |
| |
|
|
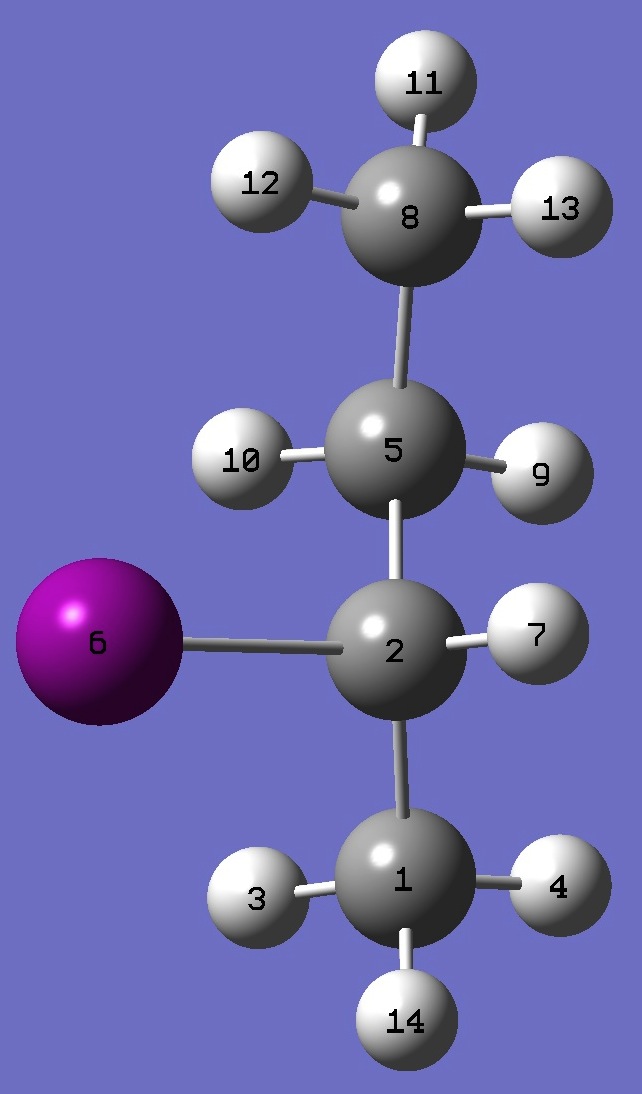
|
C
C,1,B1
H,1,B2,2,A1
H,1,B3,2,A2,3,D1,0
C,2,B4,1,A3,3,D2,0
I,2,B5,1,A4,5,D3,0
H,2,B6,1,A5,5,D4,0
C,5,B7,2,A6,1,D5,0
H,5,B8,2,A7,1,D6,0
H,5,B9,2,A8,1,D7,0
H,8,B10,5,A9,2,D8,0
H,8,B11,5,A10,2,D9,0
H,8,B12,5,A11,2,D10,0
H,1,B13,2,A12,5,D11,0
|
|
|
| ropt (1) |
ropt (2) |
|
|
|
B1=1.52317883
B2=1.09380661
B3=1.09692919
B4=1.5248739
B5=2.1868059
B6=1.09405026
B7=1.52658441
B8=1.09968519
B9=1.09637146
B10=1.09371027
B11=1.09249263
B12=1.09504347
B13=1.09219651
A1=110.7211899
A2=108.77743318
A3=112.72215221
A4=109.16659908
A5=110.46794918
A6=114.9341761
A7=105.77939872
A8=109.25393226
A9=110.54331757
A10=111.18154005
A11=110.5941325
A12=111.7530916
D1=119.10262464
D2=-59.4742902
D3=123.75960581
D4=-123.25029203
D5=-172.60449607
D6=-52.18191693
D7=62.50058581
D8=174.45294883
D9=-65.32857475
D10=54.82150498
D11=179.5036121
|
B1=1.51755814
B2=1.08925784
B3=1.09199479
B4=1.51928659
B5=2.15434962
B6=1.08970337
B7=1.52033657
B8=1.09499216
B9=1.09202986
B10=1.08865417
B11=1.08825679
B12=1.09019032
B13=1.08753091
A1=110.57528753
A2=109.05214861
A3=112.41448937
A4=109.0168063
A5=110.67029242
A6=114.53244201
A7=106.13645621
A8=109.03577363
A9=110.72683169
A10=111.05026754
A11=110.67841065
A12=111.67273419
D1=119.15450508
D2=-59.51459237
D3=123.20595779
D4=-123.62854331
D5=-173.81966205
D6=-53.14284984
D7=61.67101602
D8=174.84352295
D9=-64.96345319
D10=55.08230146
D11=179.68922205
|
|
|
|
|
|