|
| |
|
|
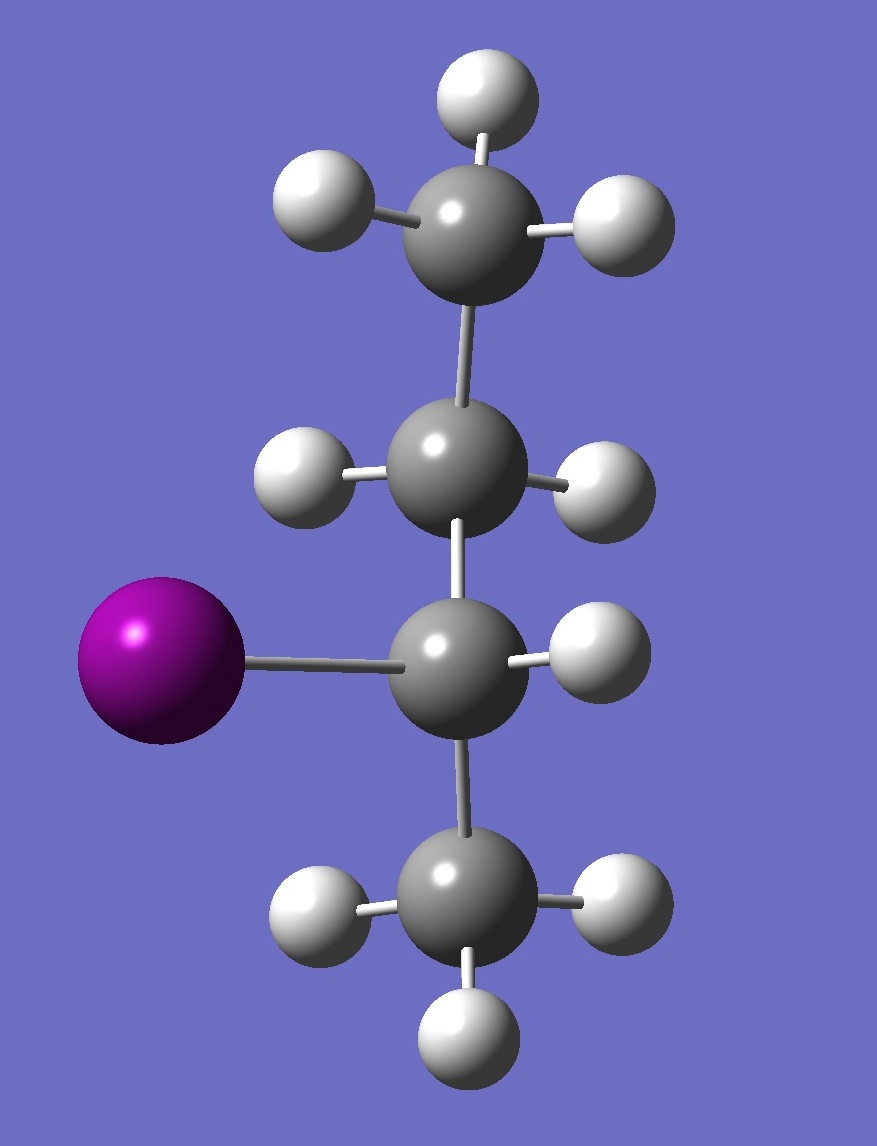
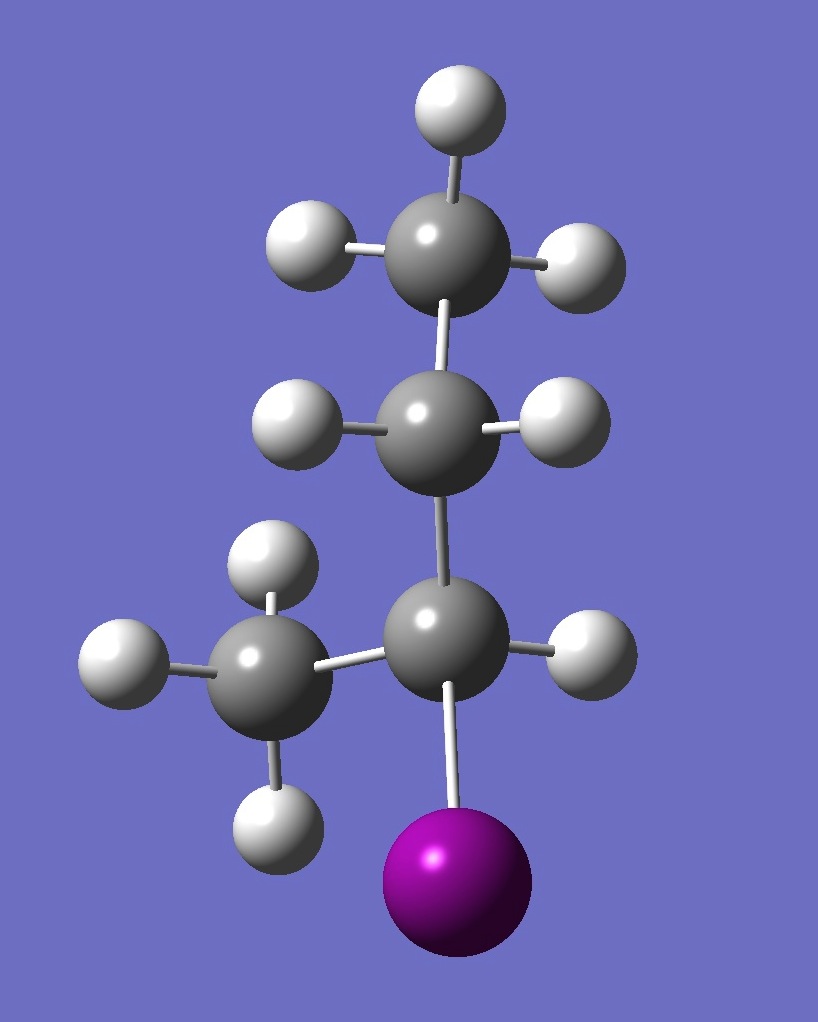
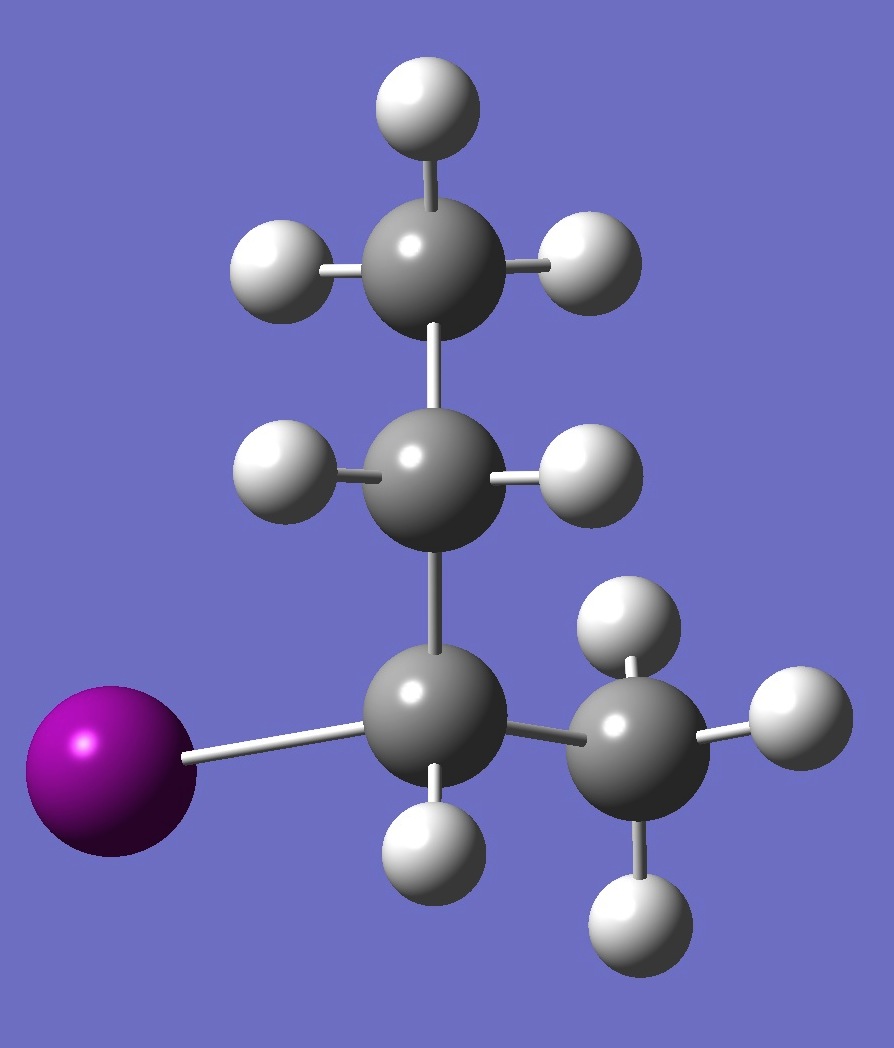
| Table 2. G'-2-Iodobutane ropt (1) = MP2/6-311+G(d,p) optimization, and ropt (2) = MP2/6-311+G(3df,3pd) structure parameters
(Å and degrees). |
| |
|
|
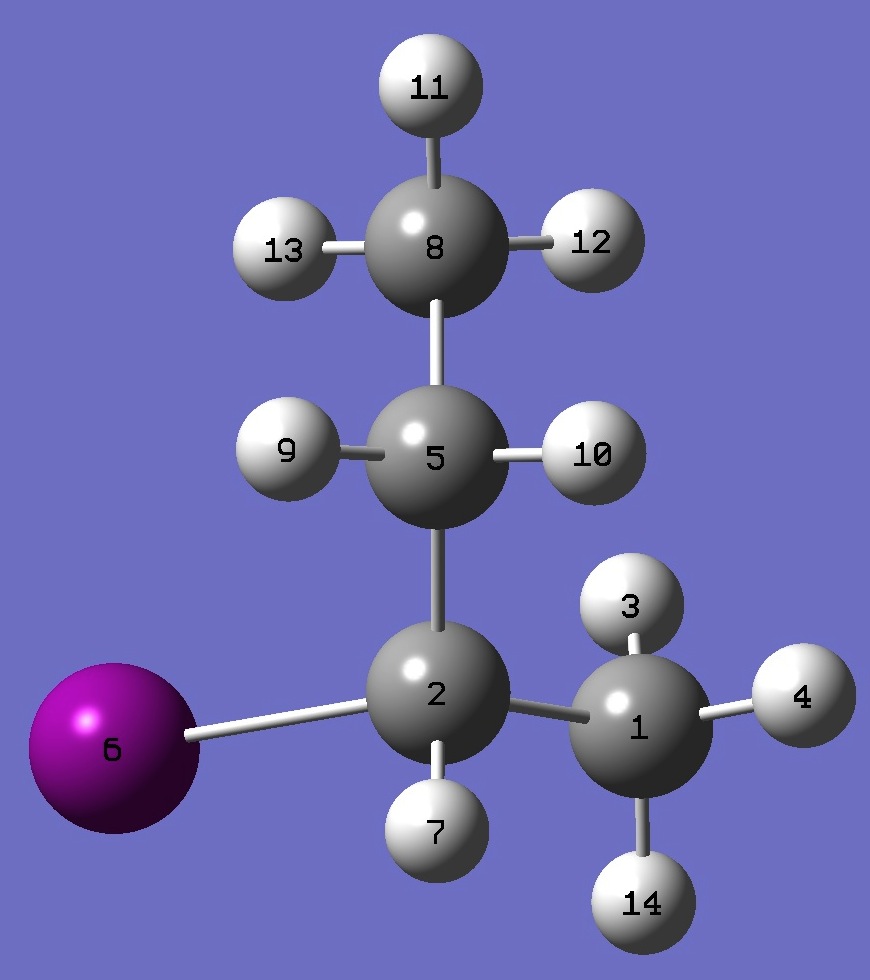
|
C
C,1,B1
H,1,B2,2,A1
H,1,B3,2,A2,3,D1,0
C,2,B4,1,A3,3,D2,0
I,2,B5,1,A4,5,D3,0
H,2,B6,1,A5,5,D4,0
C,5,B7,2,A6,1,D5,0
H,5,B8,2,A7,1,D6,0
H,5,B9,2,A8,1,D7,0
H,8,B10,5,A9,2,D8,0
H,8,B11,5,A10,2,D9,0
H,8,B12,5,A11,2,D10,0
H,1,B13,2,A12,5,D11,0
|
|
|
| ropt (1) |
ropt (2) |
|
|
|
B1=1.52310023
B2=1.09223902
B3=1.09759538
B4=1.52861697
B5=2.18947412
B6=1.09263375
B7=1.52758481
B8=1.09477801
B9=1.10004137
B10=1.09365685
B11=1.0932972
B12=1.09335379
B13=1.09234953
A1=111.75825536
A2=108.68852526
A3=114.10686128
A4=109.79741483
A5=109.87777404
A6=115.37472811
A7=109.18483904
A8=105.96852971
A9=110.07310062
A10=111.59827742
A11=110.97858872
A12=111.14984883
D1=119.69869094
D2=-65.79225693
D3=125.39095308
D4=-122.76559199
D5=55.84106123
D6=-179.78548805
D7=-65.14030314
D8=178.01891734
D9=-62.52409799
D10=58.46838694
D11=173.30791606
|
B1=1.51785477
B2=1.08769552
B3=1.09262469
B4=1.52336002
B5=2.15648335
B6=1.08813492
B7=1.52156264
B8=1.09033412
B9=1.09510242
B10=1.08850508
B11=1.08841388
B12=1.08916027
B13=1.08754587
A1=111.46803697
A2=109.00439881
A3=113.94605993
A4=109.6367409
A5=110.0430286
A6=115.03692124
A7=109.01387852
A8=106.22312037
A9=110.24015007
A10=111.56105514
A11=110.8618978
A12=111.08401858
D1=119.73797684
D2=-65.04369414
D3=124.70453539
D4=-123.08429058
D5=56.95360797
D6=-179.05009639
D7=-64.18469321
D8=178.33309543
D9=-62.04576769
D10=58.73605952
D11=174.2901898
|
|
|
|
|
|